China-based Jacobio Pharma (HKG: 1167) announced the first patient dosing of a Phase I/IIa study for its self-developed pan-KRAS inhibitor, JAB-23E73, in the United States. The dose-escalation study of the drug in China is also progressing as planned.
KRAS Mutation Impact
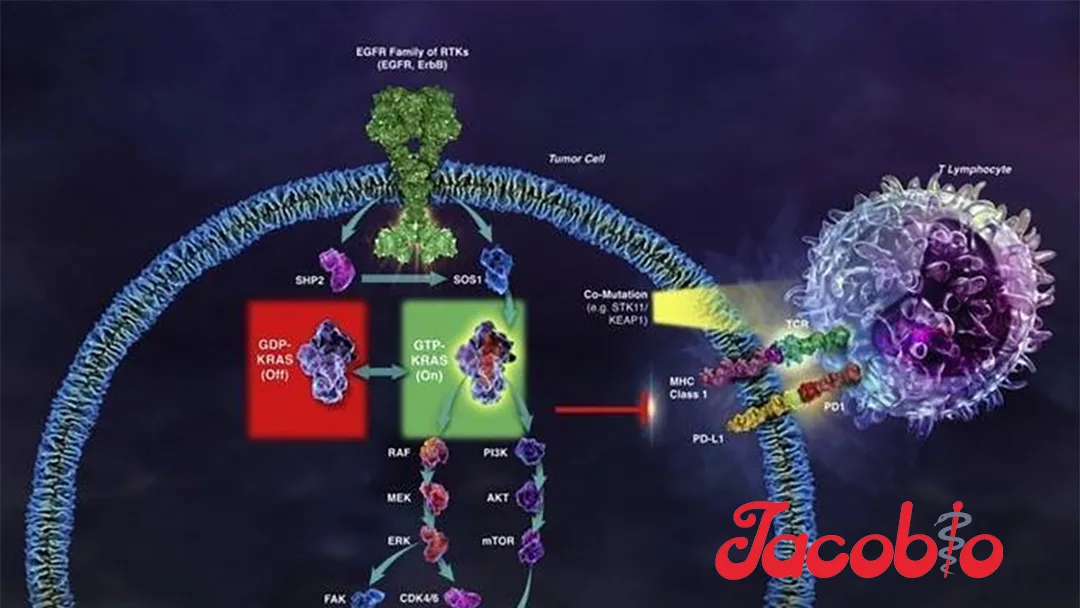
KRAS is one of the most frequently mutated oncogenes in cancer, with approximately 23%-25% of cancer patients harboring KRAS mutations. These mutations are prevalent in pancreatic cancer (~90%), colorectal cancer (~40%), and non-small cell lung cancer (~30%), among other malignancies. Globally, around 2.7 million new KRAS-mutated cancer cases are diagnosed annually, highlighting a significant unmet medical need.
JAB-23E73: A Promising Pan-KRAS Inhibitor
JAB-23E73 is a highly selective oral KRAS inhibitor that targets both the active and inactive states of KRAS while showing no significant inhibition of HRAS or NRAS. Preclinical studies have demonstrated its favorable pharmacokinetic profile and potent antitumor activity. Jacobio stated that it will continue advancing the global clinical development of JAB-23E73 to address this critical need in oncology.-Fineline Info & Tech