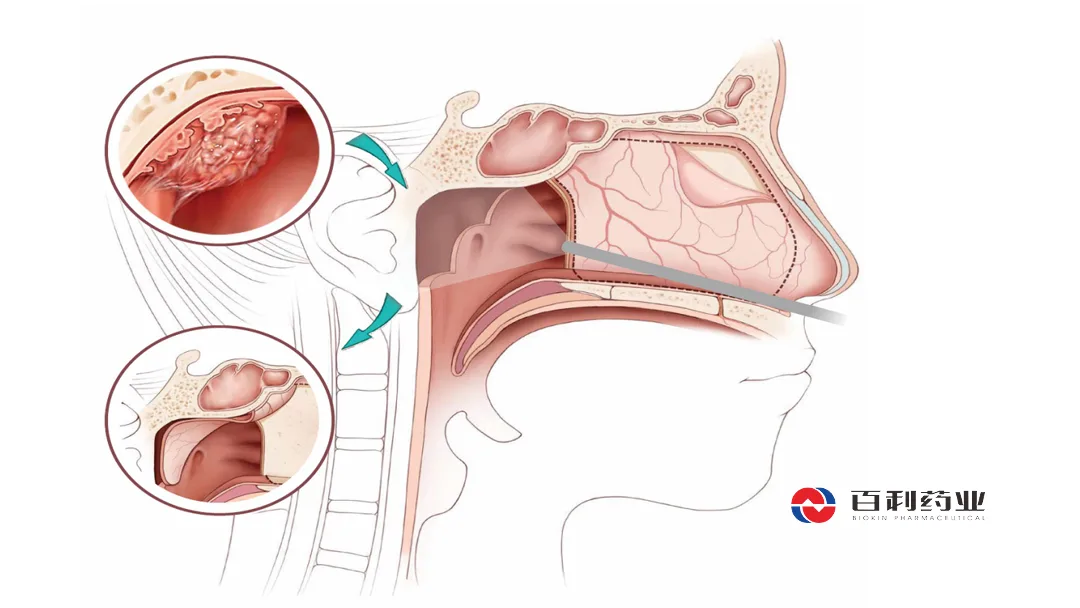
China-based Sichuan Biokin Pharmaceutical Co., Ltd (SHA: 688506) announced that its Phase III clinical study for iza-bren, an EGFR×HER3 bispecific antibody drug conjugate (ADC), has met the primary endpoint in an interim analysis. The study focused on recurrent/metastatic nasopharyngeal carcinoma (NPC) that has failed PD-1/PD-L1 monoclonal antibody therapy and progressed after ≥2 prior chemotherapy lines, including ≥1 platinum-based regimen.
Iza-bren: A First-in-Class ADC
Iza-bren is the world’s first and only EGFR×HER3 bispecific ADC to reach Phase III clinical development. The drug is currently the subject of over 40 clinical studies across multiple tumor types.
Regulatory Recognition
To date, iza-bren has received Breakthrough Therapy Designations from China’s National Medical Products Administration (NMPA) for five different indications.-Fineline Info & Tech