Wepon Medical Holding Group Co., Ltd. (SHE: 002082) announced that it has received clinical approval from the National Medical Products Administration (NMPA) for its in-house developed WP107 (huperzine A), an acetylcholinesterase inhibitor. The company plans to initiate a randomized, double-blinded, single-dosed, placebo parallel controlled study to evaluate the pharmacokinetics and safety of the drug in generalized myasthenia gravis (gMG).
Drug Background and Mechanism
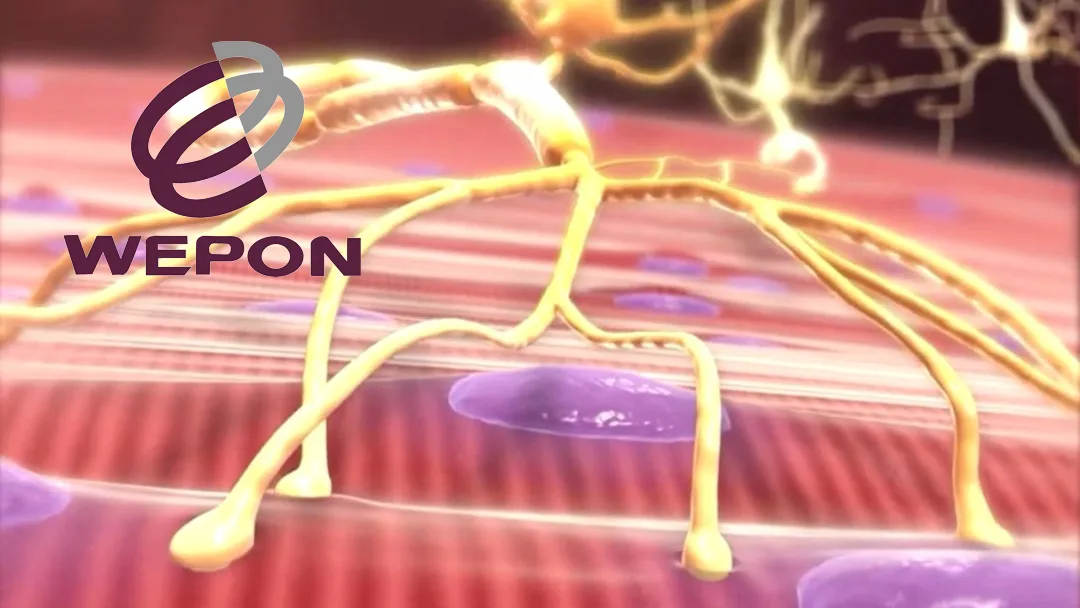
WP107 is a novel oral preparation of huperzine A, which works as an acetylcholinesterase inhibitor. This mechanism helps increase acetylcholine levels at neuromuscular junctions, potentially improving muscle strength and function in patients with gMG.
Regulatory Milestones
In December 2023, WP107 was granted Orphan Drug Designation (ODD) by the US FDA for gMG. This designation facilitates the development and approval of drugs for rare diseases. Additionally, the FDA cleared the drug for clinical trials in the US in January of this year.-Fineline Info & Tech