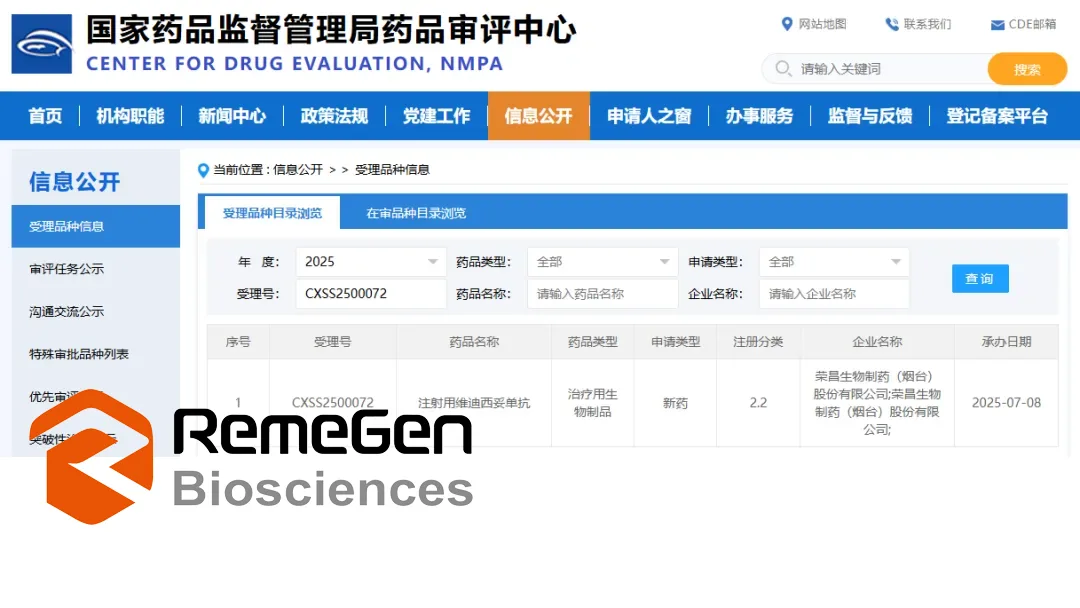
RemeGen Ltd (HKG: 9995) announced that the National Medical Products Administration (NMPA) in China has accepted the application for a new indication of its drug candidate disitamab vedotin (RC48). The application seeks approval for the drug’s use in combination with toripalimab for first-line treatment of HER2-expressing (IHC 1+/2+/3+) locally advanced or metastatic urothelial carcinoma (UC).
Drug Background
Disitamab vedotin is a home-grown antibody drug conjugate (ADC) that received conditional market approval in China in June 2021. Initially approved as a third-line treatment for HER2-positive gastric cancer (GC), it gained a second approval in December 2021 for second-line urothelial carcinoma (UC). Most recently, in April of this year, it received a third approval for treating HER2-positive advanced breast cancer with liver metastases.-Fineline Info & Tech