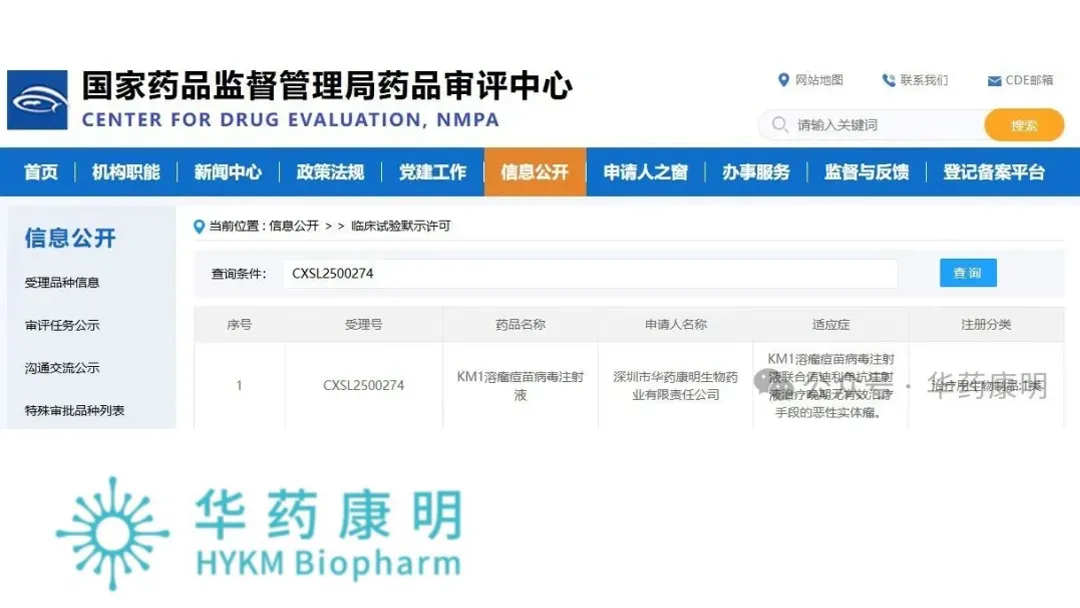
Shenzhen-based oncolytic virus specialist HKYM Biopharma has announced receiving the green-light from the National Medical Products Administration (NMPA) to proceed with a Phase Ib/II study for its in-house developed oncolytic vaccinia virus product, KM1. This approval paves the way for testing the novel therapeutic in patients with advanced malignant solid tumors who currently lack effective treatment options.
KM1: A Promising Oncolytic Virus Drug
KM1, developed on the vaccinia virus platform, has demonstrated robust selective tumor-killing capabilities and the potential to activate the body’s anti-tumor immune response. This innovative approach represents a significant step forward in the field of oncolytic virus therapy, offering a new avenue for patients with limited treatment alternatives.
Preliminary Clinical Results Show Promise
In preliminary clinical exploration, KM1 has exhibited favorable safety profiles, with some patients achieving complete remission (CR). These early results highlight the drug’s potential to provide new therapeutic hope for cancer patients, underscoring the importance of further clinical development to evaluate its efficacy and safety in a broader patient population.-Fineline Info & Tech