Suzhou-based Vigonvita Life Sciences Co., Ltd., announced that it has received marketing approval from the National Medical Products Administration (NMPA) for its simenafil (TPN171), a Category 1 drug co-developed with the Shanghai Institute of Materia Medica. The drug is approved for the treatment of erectile dysfunction (ED).
Simenafil’s Mechanism and Advantages
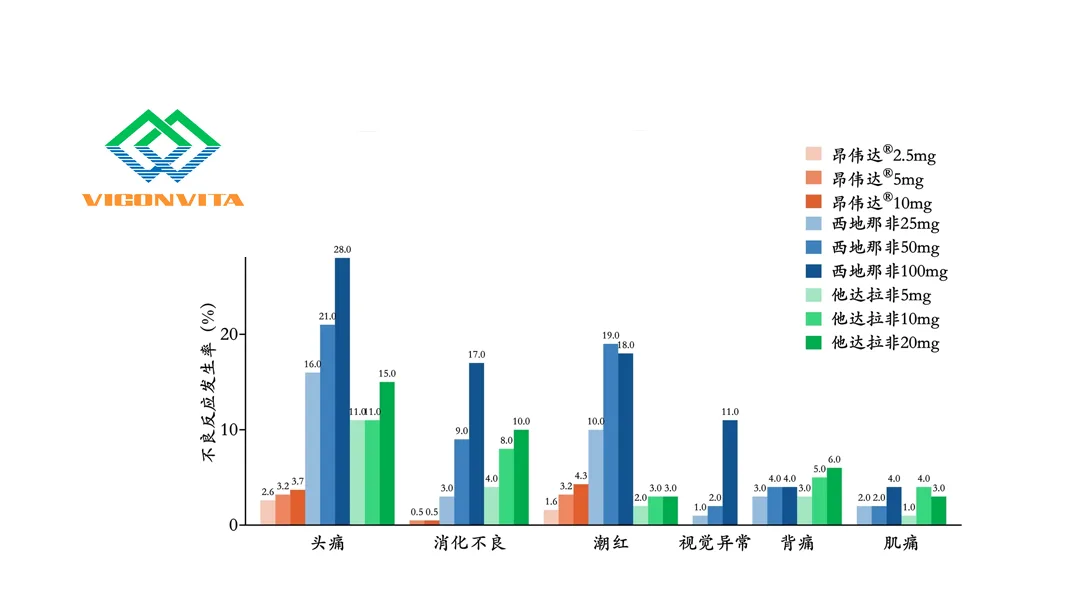
Simenafil is a novel, highly potent, and selective phosphodiesterase-5 (PDE5) inhibitor. It features a distinct chemical structure and offers significant clinical efficacy with a favorable safety profile. This innovative drug demonstrates broad patient applicability across diverse usage scenarios and has potential to be the best-in-class therapeutic option for ED patients.
On-Demand Use and Special Populations
Simenafil takes effect within 30 minutes of oral administration (on-demand use) and has an 8-11 hour half-life, aligning with natural circadian rhythms. It is suitable for special populations, including elderly patients and those with mild-to-moderate hepatic impairment, as well as all stages of renal impairment (mild to severe). Additionally, the drug maintains efficacy when consumed with moderate alcohol.-Fineline Info & Tech