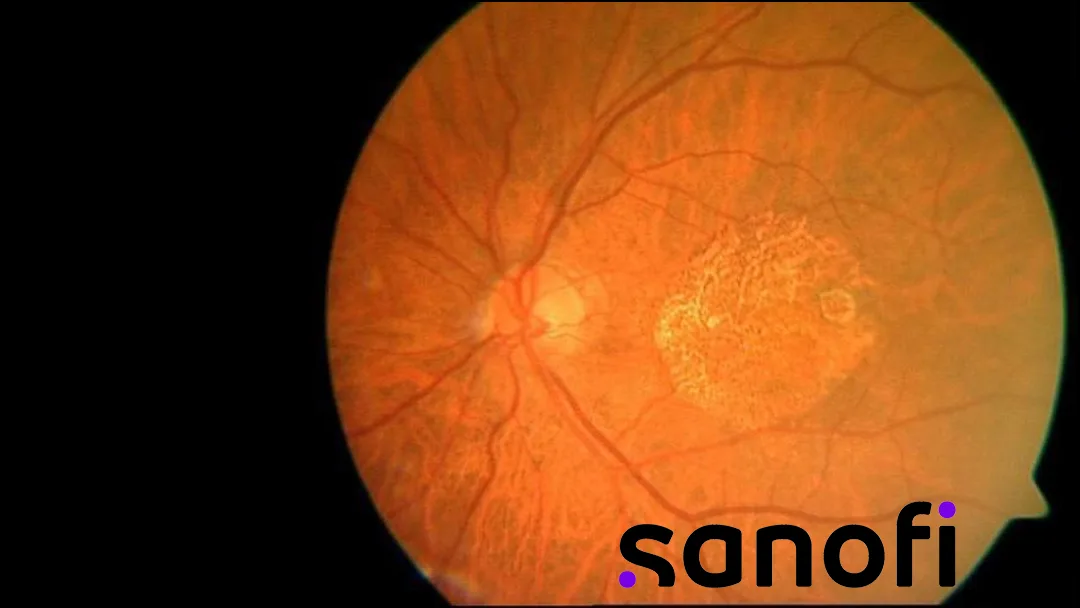
France-based Sanofi (NASDAQ: SNY) announced that its one-time intravitreal gene therapy SAR446597 has received FDA Fast Track designation for geographic atrophy secondary to age-related macular degeneration. This designation streamlines the development and review process for this sight-threatening condition.
Therapy Mechanism
SAR446597 delivers genetic instructions for two antibody fragments that target and silence C1s in the classical complement cascade and factor Bb in the alternative pathway. This approach aims to achieve durable suppression of complement-driven retinal damage following a single injection.
Clinical Development
Sanofi is preparing to initiate a Phase I/II trial to evaluate the safety, tolerability, and efficacy of SAR446597. Additionally, a related gene therapy, SAR402663, is already in Phase I/II clinical trials for neovascular AMD (NCT06660667).-Fineline Info & Tech