China-based PHASE Scientific has entered into an exclusive distribution agreement with Lumos Diagnostics to introduce the innovative FebriDx rapid test in the United States. This expands PHASE’s INDICAID respiratory portfolio and leverages its established market presence.
FebriDx Technology and Regulatory Status
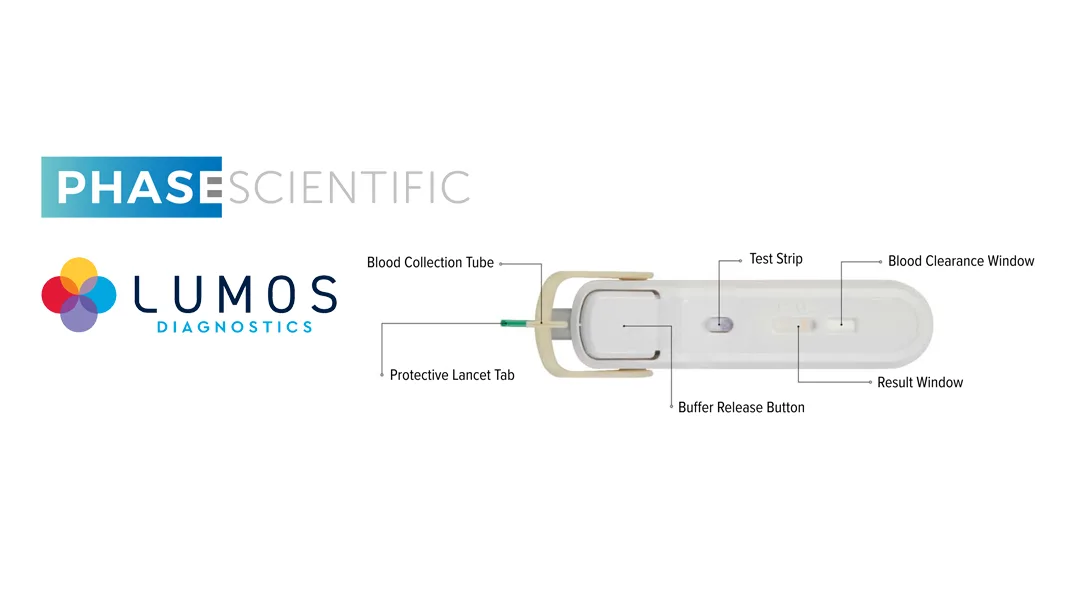
FebriDx, which has received FDA 510(k) clearance, is a point-of-care assay designed to differentiate bacterial from non-bacterial acute respiratory infections using just a single drop of blood. Results are available in approximately 10 minutes. This capability supports targeted therapy and helps reduce antibiotic misuse. A CLIA-waiver submission for the test is anticipated within the next three months.
Market Expansion Strategy
PHASE Scientific will utilize its nationwide clinical network and its proven experience of distributing over 100 million rapid tests in the U.S. to drive adoption of FebriDx. The focus will be on urgent-care and outpatient settings, where rapid and accurate diagnostic tools are highly valued.-Fineline Info & Tech