Duality Biologics (HKG: 9606) announced that the US Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) to its next-generation HER3 antibody-drug conjugate (ADC), DB-1310. The designation applies to the treatment of adult patients with advanced/unresectable or metastatic non-squamous non-small cell lung cancer (nsqNSCLC). This includes patients who have experienced disease progression during or after treatment with third-generation EGFR tyrosine kinase inhibitors and platinum-based chemotherapy, and who have EGFR exon 19 deletion or L858R mutations.
DB-1310: Next-Generation ADC
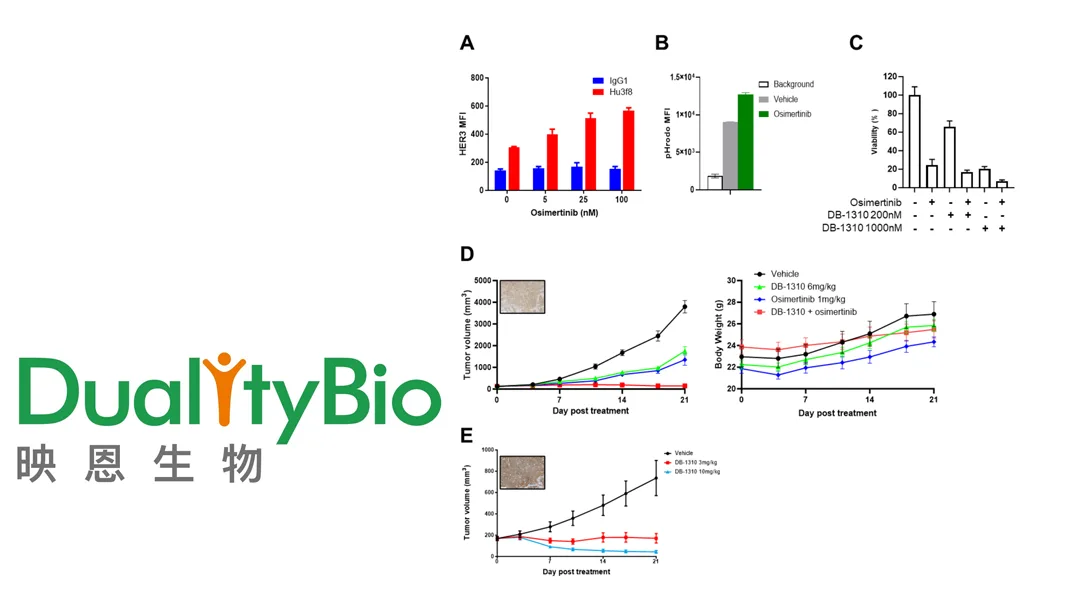
DB-1310 is a next-generation HER3-targeted ADC developed by DualityBio using its proprietary DITAC (DNA Topoisomerase Inhibitor-based ADC) technology platform. The drug has shown promising results in the first-in-human Phase I/IIa clinical study (NCT05785741), demonstrating encouraging efficacy and manageable safety in patients with advanced solid tumors who have failed standard treatments.
Clinical Progress
The Fast Track Designation underscores the FDA’s recognition of DB-1310’s potential to address significant unmet medical needs in the treatment of nsqNSCLC. This designation is expected to facilitate the development and review process, potentially accelerating the availability of this innovative therapy to patients.-Fineline Info & Tech