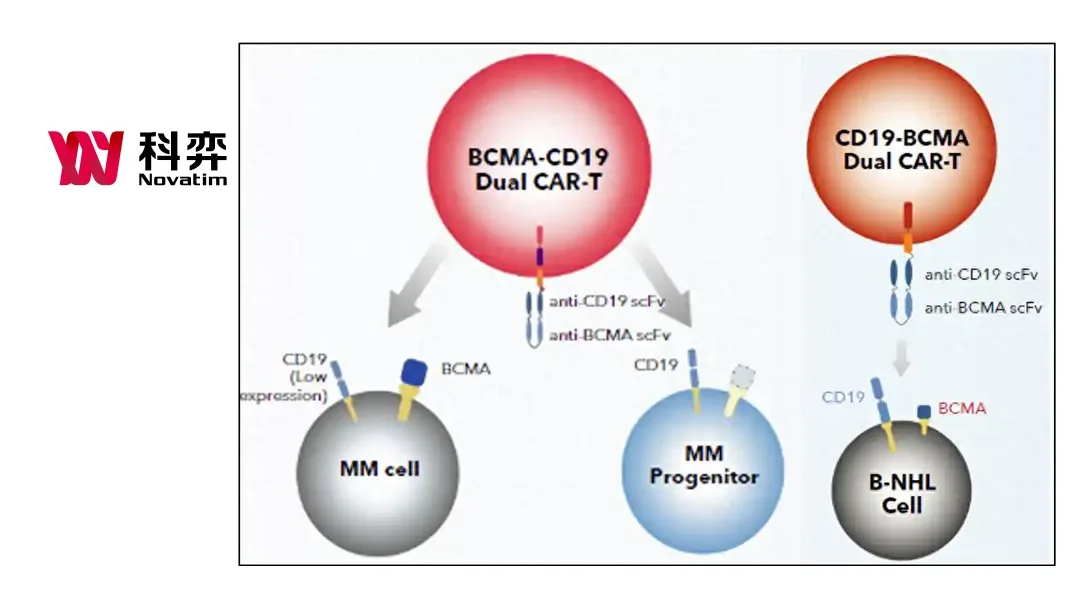
Novatim Immune Therapeutics (Zhejiang) Co., Ltd. announced a strategic cooperation with US-based biopharmaceutical company ERIGEN LLC. The partnership involves an exclusive overseas licensing agreement for Novatim’s self-developed KQ-2003, the world’s first parallel-enhanced dual-target CAR-T cell therapy targeting BCMA/CD19. This collaboration grants ERIGEN exclusive rights to develop, register, and commercialize the product globally, excluding Greater China, India, Turkey, and Russia.
License Agreement Details
Under the agreement, ERIGEN will have exclusive rights to develop “off-the-shelf CAR-T cell therapies” using the key patent structures and sequences of KQ-2003. Novatim retains complete rights to the off-the-shelf product within Greater China, setting the stage for future joint development and regional commercialization. Novatim will receive an initial milestone payment of $15 million and is eligible for up to $1.32 billion in development, registration, and commercialization milestones. Additionally, Novatim will earn up to $800 million in sales royalties based on the net sales of KQ-2003 in licensed territories.
KQ-2003 Innovation
KQ-2003 is an innovative therapy developed by Novatim using its enhanced dual-target CAR-T platform. The therapy employs parallel structural design and signaling domain optimization to significantly enhance the persistence and anti-tumor activity of CAR-T cells. This approach reduces the risk of relapse commonly associated with traditional CAR-T therapies. KQ-2003 has shown promising efficacy in registration clinical trials for relapsed/refractory multiple myeloma (RRMM) and POEMS syndrome.-Fineline Info & Tech