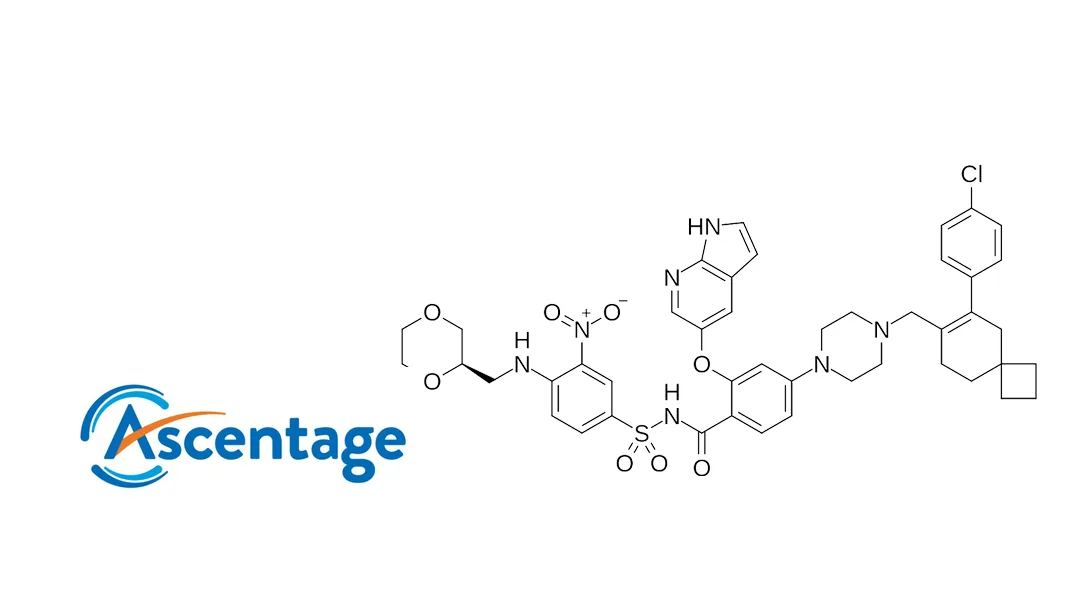
China-based Ascentage Pharma (NASDAQ: AAPG, HKG: 6855) announced today that it has entered into cooperation agreements with Sinopharm Group Co., Ltd, Shanghai Pharmaceuticals Holding Co., Ltd, and China Resources Tianjin Pharmaceutical Co., Ltd. These partnerships aim to combine the strengths of each party in R&D innovation, market development, and channel management to jointly advance the commercialization of Lisaftoclax (generic name: lisaftoclax), China’s first approved domestic innovative Bcl-2 inhibitor.
About Lisaftoclax
Lisaftoclax is a novel, orally administered, selective Bcl-2 inhibitor independently developed by Ascentage Pharma. It received marketing approval in China on July 8 for treating adult patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have received at least one prior systemic therapy, including a BTK inhibitor.
Strategic Importance
These strategic cooperation agreements are a crucial part of Ascentage Pharma’s commercialization strategy. They initiate multi-faceted collaborations to explore suitable models for pre-launch services, logistics, national distribution, market access to end hospitals and pharmacies, and new retail expansion. The goal is to accelerate the nationwide commercialization of Lisaftoclax efficiently, enabling this innovative Chinese drug to benefit more patients sooner.-Fineline Info & Tech