China-based Fosun Pharmaceutical (SHA: 600196) announced that its controlling subsidiary, Fosun Pharma Industrial, has entered into a licensing agreement with Newco United Technology. This agreement grants Fosun Pharma exclusive rights to develop, register, manufacture, and commercialize the investigational drug AR1001 in mainland China, Hong Kong, and Macau. The licensed indications include Alzheimer’s disease (AD) and other neurological disorders.
AR1001: Mechanism and Potential
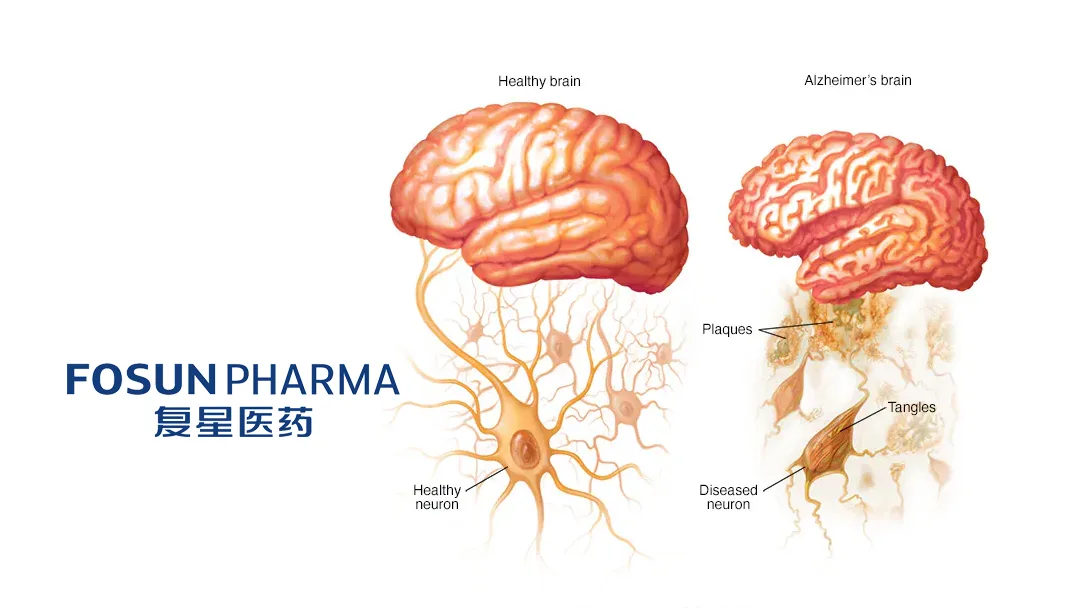
AR1001 is an oral small molecule PDE5 inhibitor designed to slow the progression of Alzheimer’s disease. It acts as a potent and highly selective inhibitor of phosphodiesterase-5 (PDE-5). Preclinical and clinical studies suggest that AR1001 can clear amyloid plaques, inhibit abnormal Tau protein phosphorylation and inflammatory responses, and provide neuroprotective effects. Currently in a global multi-center Phase III clinical trial for early AD, AR1001 has demonstrated good blood-brain barrier permeability and safety. No drugs targeting this specific mechanism have been approved globally for AD treatment to date.
Financial and Strategic Terms
Under the agreement, Fosun will make an upfront and regulatory milestone payment to Newco United Technology, not exceeding RMB 150 million. Additional payments will be based on sales milestones and tiered sales royalties from net sales of localized products. The agreement also includes supply arrangements, preferred negotiation rights, and provisions for future rights discussions in several Southeast Asian countries.-Fineline Info & Tech