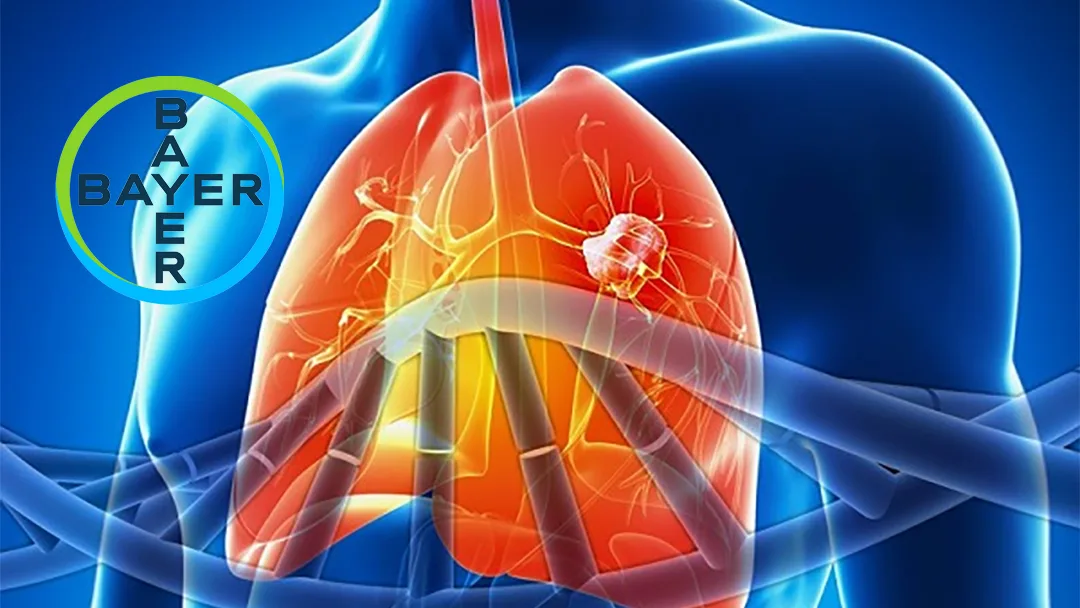
German pharmaceutical giant Bayer AG (ETR: BAYN) announced on July 28, 2025, that the marketing application for its targeted therapy, Sevabertinib, has been accepted by China’s National Medical Products Administration (NMPA) Center for Drug Evaluation (CDE) and granted priority review status. Sevabertinib is an oral small-molecule tyrosine kinase inhibitor (TKI) for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) harboring HER2 activating mutations. These patients have previously received at least one systemic therapy. The marketing application in China was submitted concurrently with filings in the United States and other global countries.
Drug Mechanism and Development
Sevabertinib targets HER2 exon 20 insertion mutations and point mutations, demonstrating high selectivity for both mutant HER2 and EGFR. The application is based on positive results from the SOHO-01 Phase I/II trial. In this trial, the drug showed an objective response rate of 71% in advanced NSCLC patients who had received prior systemic therapy but no prior HER2-targeted TKI. The safety profile was favorable and consistent with previous reports.
Previous Designation
Previously, Sevabertinib had been granted Breakthrough Therapy Designation by the CDE, underscoring its potential to address significant unmet medical needs in this patient population.-Fineline Info & Tech