China-based BrightGene Bio-Medical Technology Co., Ltd. (SHA: 688166) and its affiliated companies announced a collaboration and development agreement with China Resources Sanjiu Medical & Pharmaceutical Co., Ltd. (SHE: 000999). The partnership focuses on the R&D, registration, manufacturing, and commercialization of BGM0504 Injection in mainland China (excluding Hong Kong, Macau, and Taiwan). BrightGene will grant CR Sanjiu exclusive commercialization and collaborative development rights for the product.
BGM0504: A Promising Dual Agonist
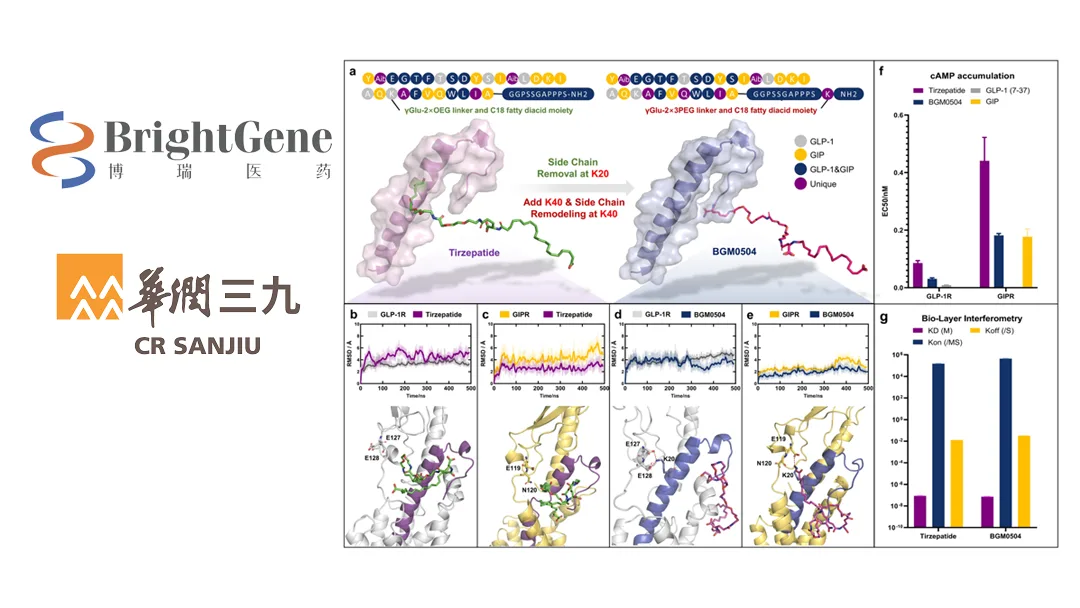
BGM0504 is a self-developed glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor dual agonist. As a Class 1 innovative chemical drug not yet marketed in or outside of China, BGM0504 Injection activates downstream GIP and GLP-1 pathways. It has the potential to control blood sugar, promote weight loss, and treat non-alcoholic steatohepatitis (NASH). The product has completed two Phase II clinical trials in China, achieving expected outcomes, and is currently in Phase III trials.
Collaboration Framework
Under the agreement, BrightGene will receive up to RMB 282 million in milestone payments based on clinical and regulatory progress. Post-launch, BrightGene will also receive service fees based on sales and, under certain conditions, additional sales milestone payments.
Joint Committees for Project Execution
To ensure the effective implementation of the project, a joint development committee and a commercialization committee will be established. These committees will oversee the project’s progress and facilitate collaboration between the two companies.-Fineline Info & Tech