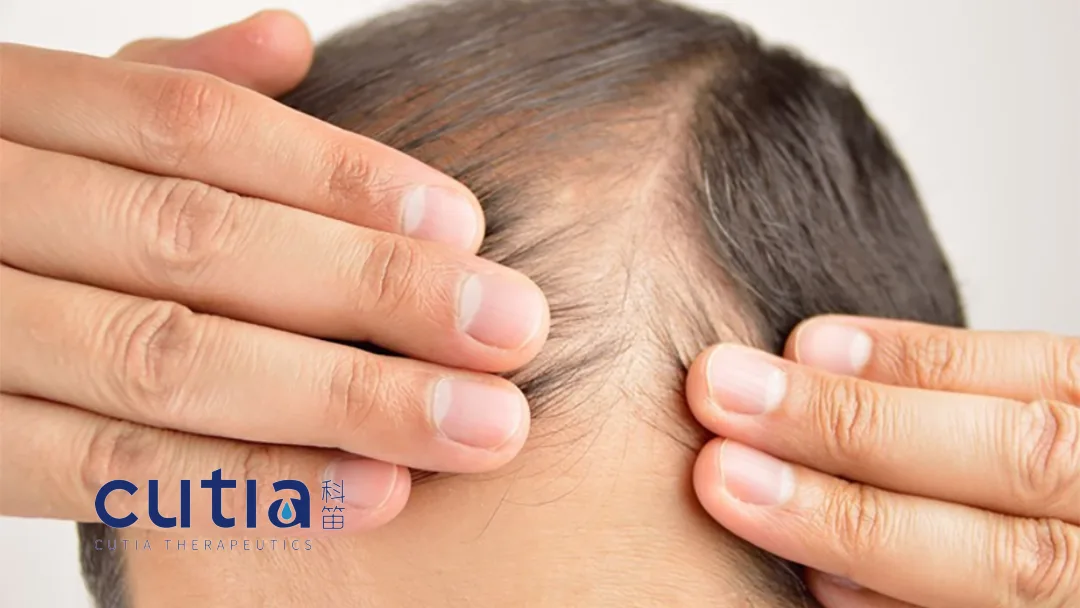
China-based dermatology specialist Cutia Therapeutics (HKG: 2487) announced today that its CU-40102 (finasteride topical spray) has received approval in Hong Kong for the treatment of androgenetic alopecia. This marks a significant milestone in providing new therapeutic options for patients suffering from hair loss.
Mechanism of Action
Finasteride, the active ingredient in CU-40102, is a specific competitive inhibitor of type II 5α-reductase. It works by inhibiting the conversion of testosterone to dihydrotestosterone (DHT) in the scalp, a key contributor to androgenetic alopecia. Unlike oral finasteride, CU-40102’s topical formulation allows for direct and precise application to the scalp, potentially reducing systemic exposure and associated side effects.
Advantages of Topical Formulation
The topical delivery system of CU-40102 offers several advantages over oral administration. By applying the medication directly to the scalp, patients may experience improved localized efficacy and a lower risk of systemic side effects. This targeted approach aligns with the growing demand for effective and user-friendly treatments for hair loss.-Fineline Info & Tech