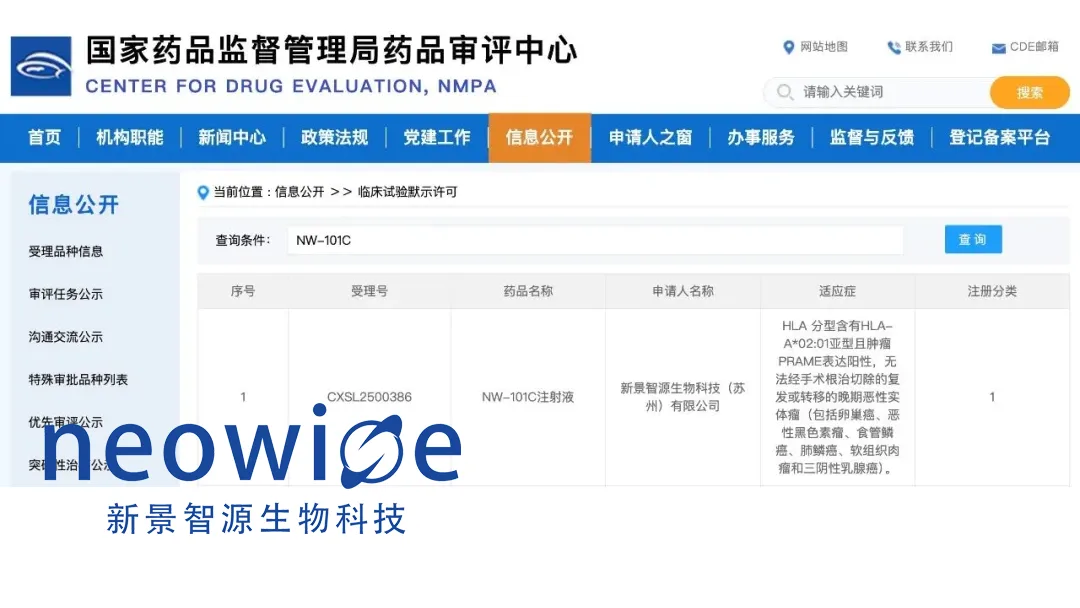
China-based Neowise Biotechnology (Suzhou) Co., Ltd. announced that its self-developed PRAME-targeting autologous TCR-T cell therapy candidate, NW-101C injection, has received implied clinical trial approval from China’s National Medical Products Administration (NMPA). This marks the company’s first product to enter registration clinical trials and represents the first PRAME-targeting TCR-T cell therapy in China to advance into clinical research.
Product Profile
NW-101C injection targets the tumor antigen PRAME, which is highly and specifically expressed in various solid tumors while being largely absent in normal tissues. This selective targeting enhances the therapy’s potential to effectively combat cancer cells while minimizing impact on healthy tissues.
Preclinical Results
In preclinical studies, NW-101C demonstrated robust anti-tumor activity and a favorable safety profile. The approved indications include patients with HLA-A*02:01-positive, PRAME-expressing, unresectable recurrent or metastatic advanced malignant solid tumors. Specific conditions covered are ovarian cancer, malignant melanoma, esophageal squamous cell carcinoma, lung squamous cell carcinoma, soft tissue sarcoma, and triple-negative breast cancer.
Company Overview
Neowise Biotechnology is an innovative company dedicated to developing TCR-T immunocell therapies for solid tumors. The company has established a high-throughput, high-sensitivity target antigen-TCR discovery platform. With multiple clinical-stage pipelines, Neowise has observed positive efficacy and good safety in several trials, positioning itself as a promising player in the field of cancer immunotherapy.-Fineline Info & Tech