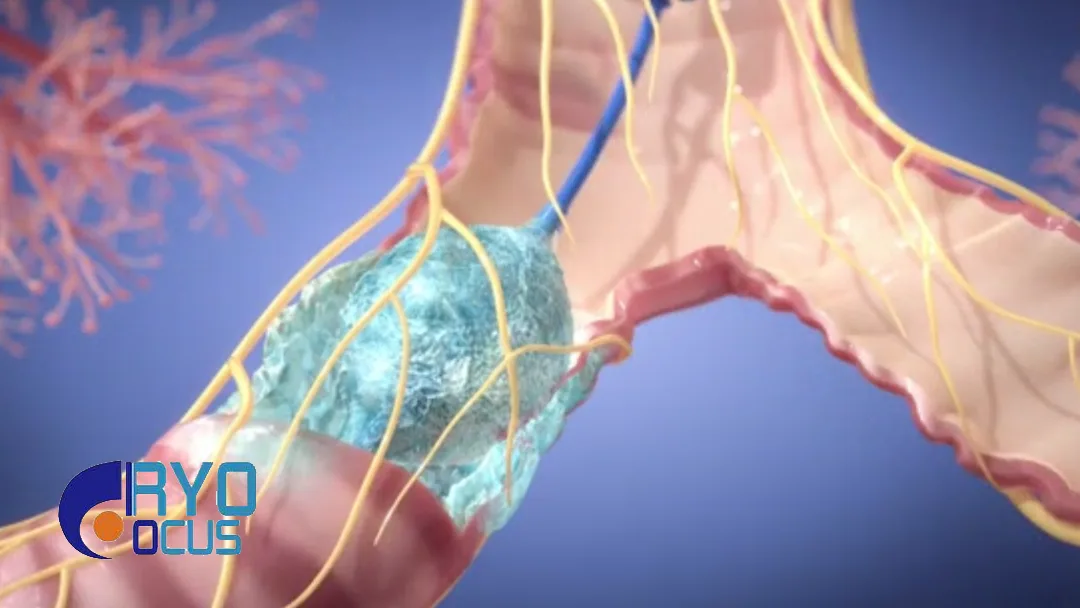
Cryofocus Medtech (Shanghai) Co., Ltd. (HKG: 6922) announced that it has received approval from China’s National Medical Products Administration (NMPA) for the disposable cryoprobe, a key component of the company’s Cryoadhesion System. This approval further optimizes the commercialization pathway for the respiratory interventional product.
Product Features
The Cryoadhesion System utilizes subcritical refrigeration technology and controlled pressure heat transfer for rapid cryoadhesion. It is designed for the cryoremoval of foreign bodies, mucous, blood clots, and necrotic tissues in the airway. Additionally, it facilitates cryobiopsy of bronchial and lung tissues.
Commercialization and Applications
With the NMPA approval, the Cryoadhesion System’s commercialization process is enhanced. The cryoprobe, available in both disposable and re-sterilizable forms, offers comprehensive solutions for relevant clinical indications. This technology represents a significant advancement in respiratory interventional procedures.-Fineline Info & Tech