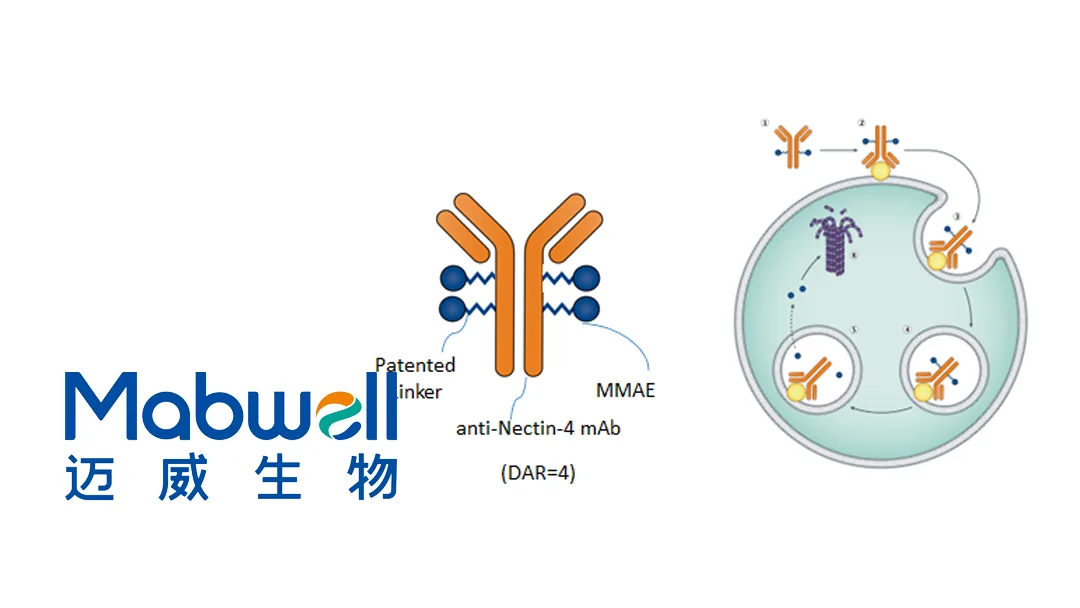
China-based Mabwell (Shanghai) Bioscience Co., Ltd (SHA: 688062) announced the first patient dosing in the U.S. for a clinical study of its 9MW2821 (Bulumtatug Fuvedotin) for triple-negative breast cancer (TNBC) patients previously treated with an antibody-drug conjugate (ADC). This marks the first overseas clinical study for 9MW2821.
Drug Profile
9MW2821 is a novel nectin-4-targeting ADC. The multicenter clinical study (NCT06908928) aims to evaluate the efficacy and safety of 9MW2821 in TNBC patients who have previously been treated with a taxane and a topoisomerase inhibitor payload ADC.
Clinical Need
Topoisomerase inhibitor-based ADCs (TOPi-ADCs) are emerging as a mainstream post-standard therapy option for TNBC. However, a significant proportion of patients progress after TOPi-ADC treatment, highlighting the urgent need for alternative therapies. Given the high expression of Nectin-4 in TNBC, the clinical study of 9MW2821 does not impose biomarker screening requirements. This approach has the potential to cover a broad patient population and offers a novel therapeutic option for TNBC patients.-Fineline Info & Tech