Arvinas, Inc. (NASDAQ: ARVN) and its partner Pfizer (NYSE: PFE) announced on August 8, 2025, that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for their PROTAC, vepdegestrant. The FDA has set a Prescription Drug User Fee Act (PDUFA) action date of June 5, 2026.
Drug Profile
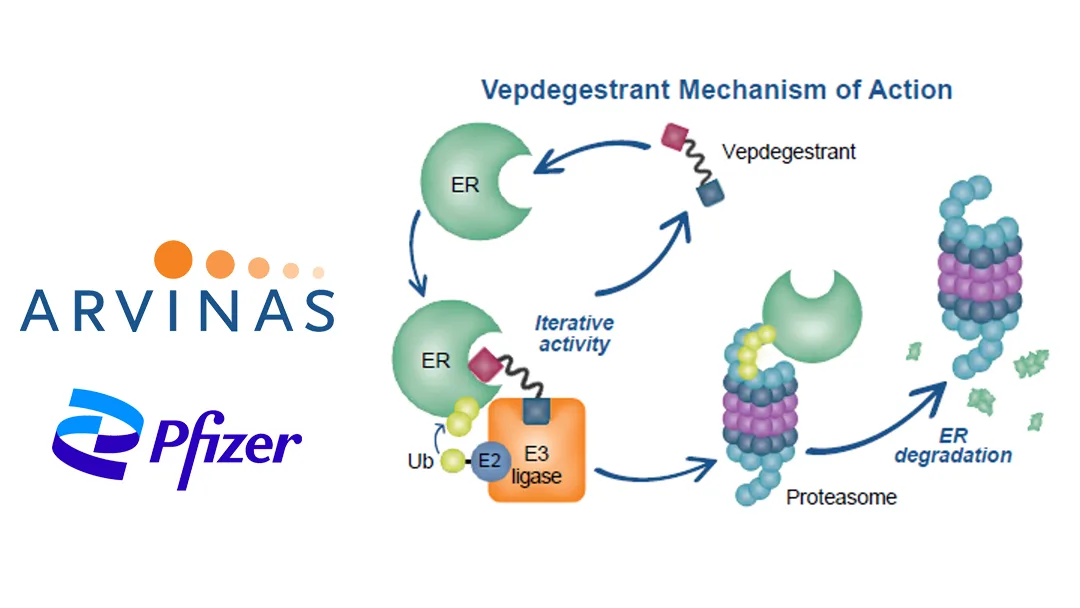
Vepdegestrant is an investigational oral PROTAC estrogen receptor degrader being jointly developed by Arvinas and Pfizer. It is intended for the treatment of patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-), ESR1-mutated advanced or metastatic breast cancer who have previously received endocrine-based therapy.
Clinical Trial Results
The NDA submission was based on data from VERITAC-2 (NCT05654623), a global, randomized Phase III clinical trial comparing vepdegestrant to fulvestrant. In March 2025, the trial produced positive top-line results, showing that vepdegestrant significantly and clinically improved progression-free survival (PFS) compared to fulvestrant.-Fineline Info & Tech