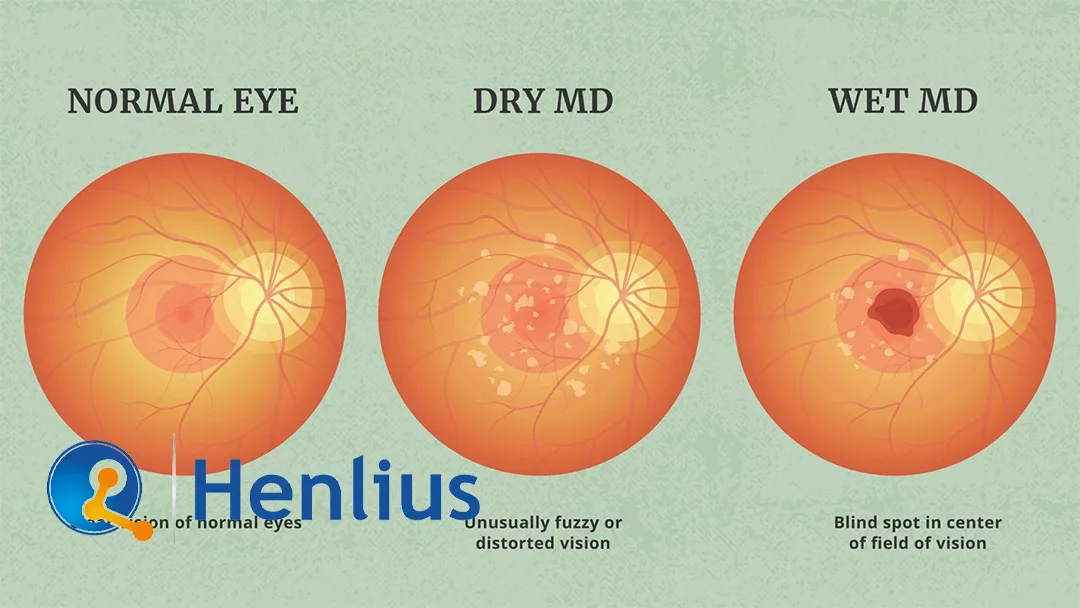
Shanghai Henlius Biotech Inc. (HKG: 2696) announced on August 13, 2025, that the marketing authorization application (NDA) for its drug HLX04-O for the treatment of wet age-related macular degeneration (wAMD) has been accepted by the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA).
Drug Profile
HLX04-O is a new ophthalmic formulation developed by Henlius based on its self-developed drug, Hanbeitai (Bevacizumab injection). The formulation, specifications, and manufacturing process were optimized for ophthalmic use while retaining the same active ingredient. It is intended for the treatment of wAMD.
Clinical Trial Results
The marketing application is supported by a non-inferiority Phase III clinical study. The results demonstrated that the mean change in best-corrected visual acuity (BCVA) from baseline at week 48 in the HLX04-O group was non-inferior to that of the Ranibizumab group, achieving the study’s primary endpoint. HLX04-O also showed a favorable safety profile with no new safety signals observed.-Fineline Info & Tech