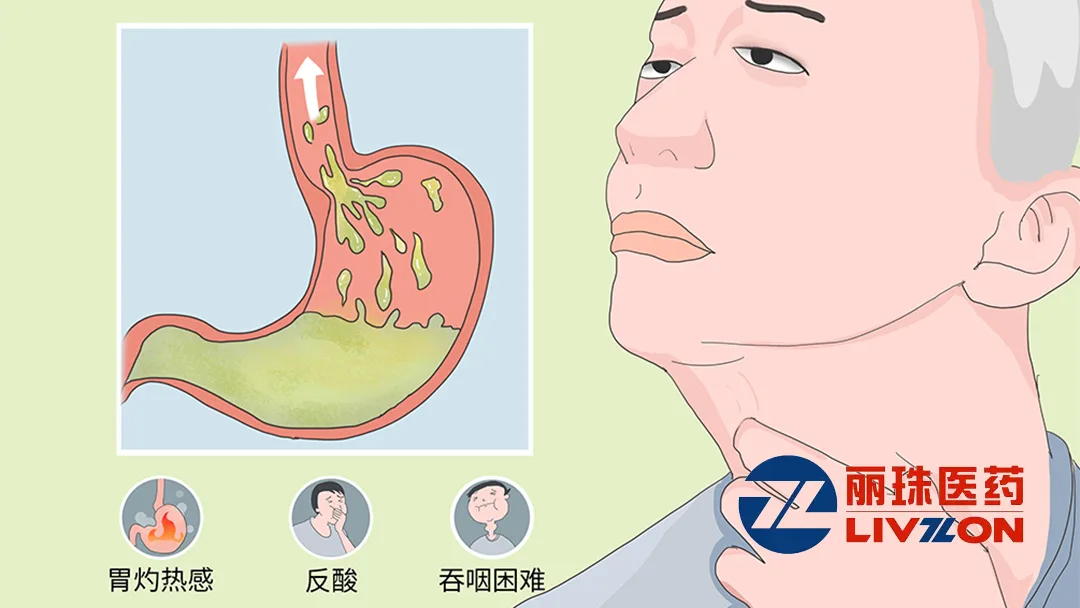
Livzon Pharmaceutical Group Inc. (HKG: 1513) announced that the drug registration application for its JP-1366 tablets for the treatment of reflux esophagitis has been received and accepted by China’s National Medical Products Administration (NMPA). This marks a significant milestone in the development of this innovative therapy.
Product Mechanism
JP-1366 tablets are an innovative potassium-competitive acid blocker (P-CAB). They work by competitively blocking the K(+) channel of H(+), K(+)-ATPase (proton pump), inhibiting the binding of K(+) to the proton pump, and suppressing gastric acid secretion. This mechanism promotes the healing of esophageal mucosa and alleviates reflux symptoms.
Clinical Advantages
The product can increase the intragastric pH value at a relatively fast rate and maintain the intragastric pH value above 4 for a prolonged period. This effectively addresses nocturnal acid breakthrough. In Phase 3 clinical trials, the esophageal mucosal healing rate of JP-1366 tablets within 8 weeks after administration was found to be non-inferior to that of Nexium (Esomeprazole Magnesium Enteric-coated Tablets).
Additional Pipeline Information
JP-1366 for injection, developed by Livzon and based on the same molecule, is currently in Phase 1 clinical trials for the indication of peptic ulcer bleeding. Notably, there is currently no P-CAB injection available on the global market.-Fineline Info & Tech