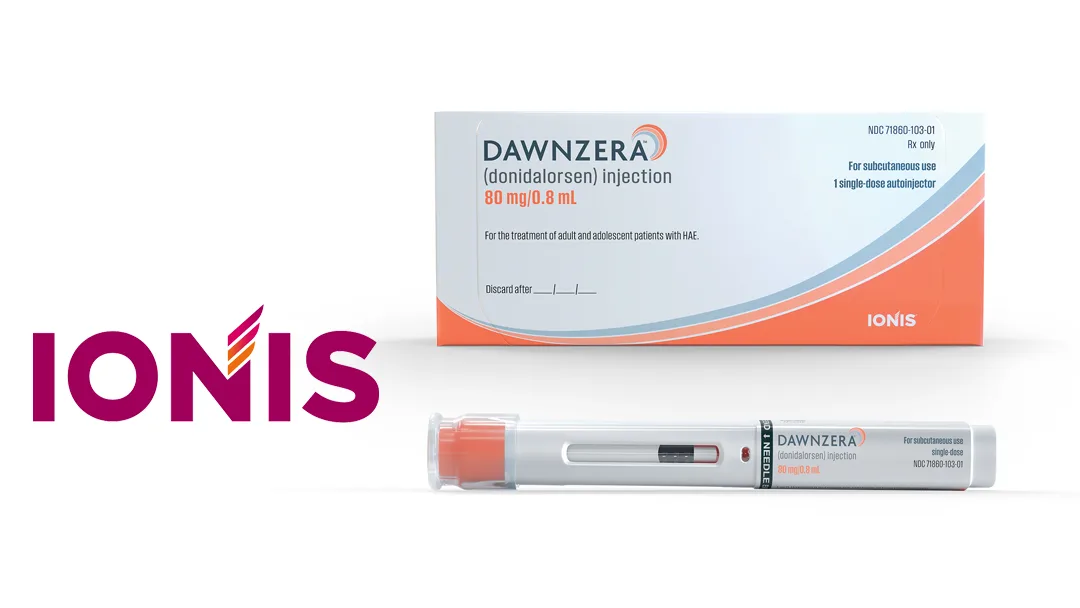
Ionis Pharmaceuticals, Inc. (NASDAQ: IONS) announced on August 22, 2025, that the U.S. Food and Drug Administration (FDA) has approved DAWNZERA (donidalorsen) for preventing attacks of hereditary angioedema (HAE) in adults and pediatric patients aged 12 and older.
First RNA-Targeted Therapy for HAE
DAWNZERA is the first and only RNA-targeted medicine approved for HAE. It targets plasma prekallikrein (PKK), a key protein involved in activating inflammatory mediators associated with acute HAE attacks.
Dosing and Availability
The 80mg dose of DAWNZERA can be self-administered via a subcutaneous autoinjector every four (Q4W) or eight (Q8W) weeks. The product will be available in the U.S. in the coming days, offering a new treatment option for HAE patients.-Fineline Info & Tech