Chinese ophthalmology specialist Ocumension Therapeutics (HKG: 1477) , a China-based pharmaceutical company, announced on August 21, 2025, that OT-301 (NCX470), one of its key pipeline drugs co-developed with Nicox S.A., has achieved its primary endpoint of non-inferiority compared to latanoprost in its second Phase III clinical trial, the Denali trial. This achievement satisfies the efficacy requirements for new drug approval in China.
Denali Trial Details
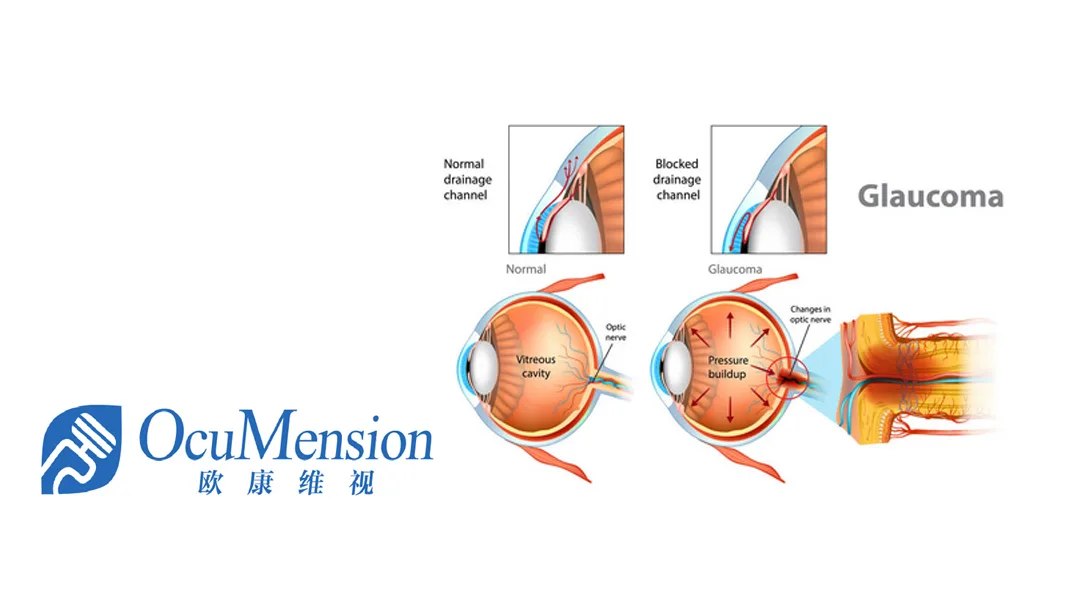
The Denali trial is a three-month, multi-regional Phase III study designed to evaluate the safety and efficacy of OT-301 ophthalmic solution (0.1% concentration) in reducing intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. The trial compared OT-301 to the current standard of care, latanoprost ophthalmic solution (0.005% concentration).
OT-301 Mechanism and Collaboration
OT-301 is a novel chemical entity developed by Nicox, designed to release both bimatoprost (an FDA-approved prostaglandin analog) and nitric oxide to reduce IOP. Ocumension secured an exclusive license from Nicox to develop, manufacture, import, export, and sell OT-301 in Greater China in December 2018. In March 2020, the exclusivity was extended to include South Korea and 12 Southeast Asian countries.-Fineline Info & Tech