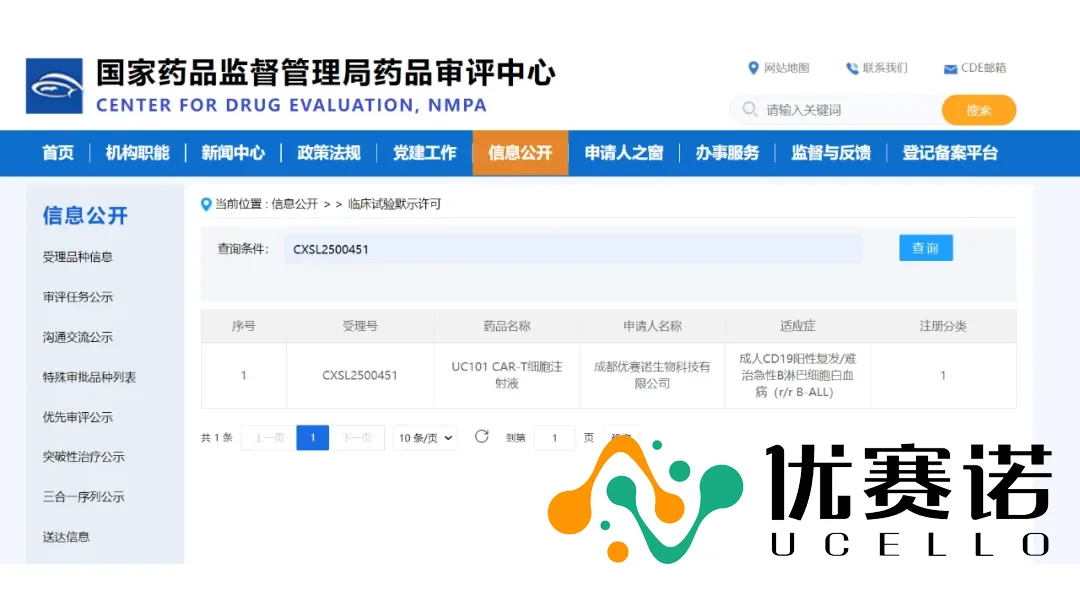
China‑based Chengdu Ucello Biotechnology Co., Ltd. announced that its Investigational New Drug (IND) application for UC101—a self‑developed, off‑the‑shelf allogeneic CAR‑T injection targeting CD19—has been officially approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA).
What Makes UC101 a Game‑Changer
UC101 is derived from newborn cord blood and employs multi‑gene editing to create a stable, off‑the‑shelf product. The platform eliminates graft‑versus‑host disease (GvHD) and host‑versus‑graft rejection (HvGR) at the source, while delivering superior in‑vivo expansion and persistence compared with conventional CAR‑T therapies.
Manufacturing Breakthroughs
UC101 is the world’s first CAR‑T therapy produced from a stable cell line that generates lentiviral vectors, eliminating the need for plasmid raw materials. The streamlined process cuts manufacturing costs by more than 70 %, and a single batch can serve over 100 patients.
Early Clinical Success in B‑ALL
In investigator‑initiated trials for B‑cell acute lymphoblastic leukemia (B‑ALL), UC101 achieved a 90 % complete remission rate with manageable adverse events. Key efficacy metrics outperformed comparable international products, underscoring the therapy’s potent B‑cell‑clearing ability.
Future Directions
Beyond B‑ALL, Chengdu Ucello plans exploratory studies of UC101 in autoimmune diseases, leveraging its robust B‑cell depletion profile. The NMPA clearance positions UC101 as a promising first‑in‑class CAR‑T candidate for both oncology and immune‑mediated disorders.
Industry Impact
The approval of UC101 marks a significant milestone for China’s burgeoning cellular therapy sector, demonstrating that off‑the‑shelf CAR‑T can be produced at scale, cost‑effective, and with an improved safety profile.-Fineline Info & Tech