Zenas BioPharma, Inc. (NASDAQ: ZBIO) and Royalty Pharma, Inc. (NASDAQ: RPRX) announced a milestone partnership that will fund the development of Obexelimab with up to $300 million in payments and a 5.5 % royalty on global net sales.
Funding Structure
| Milestone | Payment | Conditions |
|---|---|---|
| Initial | $75 million | Upon signing |
| Phase 3 INDIGO Success | $75 million | Obexelimab meets pre‑set success criteria in the Phase 3 INDIGO trial for IgG4‑related disease (expected by year‑end) |
| FDA Approval – IgG4‑Related Disease | $75 million | U.S. FDA approval granted |
| FDA Approval – Systemic Lupus Erythematosus | $75 million | U.S. FDA approval granted |
Royalty Pharma receives a 5.5 % royalty on global net sales of Obexelimab by Zenas and its affiliates, plus additional payments tied to commercialization in the partnered territories.
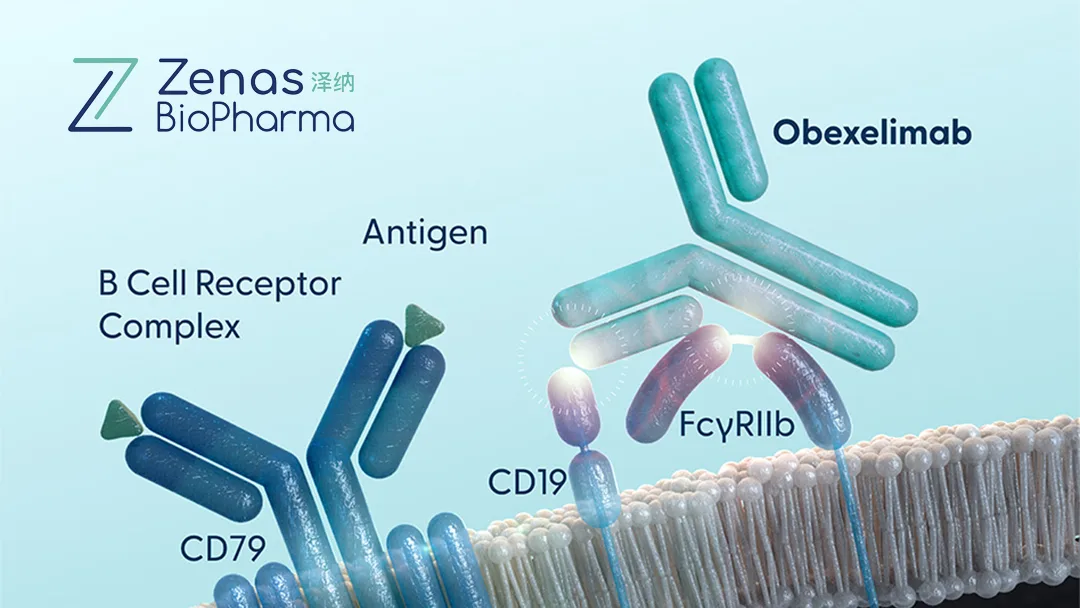
Obexelimab – A First‑In‑Class Bifunctional Antibody
- Mechanism – Humanized CD19 monoclonal antibody with an XmAb Fc domain that also binds FcγRIIb, modulating B‑cell function.
- Therapeutic Indications – IgG4‑related disease (Phase 3 INDIGO) and systemic lupus erythematosus (FDA‑approved).
- Development History – In 2021, Zenas acquired global exclusive rights from Xencor for $480 million; in 2023, BMS received exclusive rights for Japan, South Korea, Singapore, Taiwan, Hong Kong, and Australia.
Strategic Impact
- Capital Injection – The $300 million financing accelerates pre‑clinical, clinical, and regulatory milestones without diluting Zenas’ equity.
- Royalty Structure – The 5.5 % royalty aligns Royalty Pharma’s upside with Obexelimab’s commercial success, creating a long‑term partnership.
- Global Reach – Royalty Pharma’s existing distribution network and expertise in specialty therapeutics position Obexelimab for rapid market entry.