Simcere Pharmaceutical Group Limited (HKG: 2096) today announced that the National Medical Products Administration (NMPA) has accepted the new drug application (NDA) for Deunoxavir Marboxil Granules (brand name Xianlinda). The anti‑influenza formulation, jointly developed with Jiaxing AnDiCon Biotech Co., Ltd., is approved for the treatment of uncomplicated influenza A and influenza B in pediatric patients aged 2 to 11 years.
What Makes Deunoxavir Marboxil a Breakthrough
- First Pediatric Anti‑Influenza Innovation in China – The first drug of its class to complete a Phase III trial and receive regulatory approval for children.
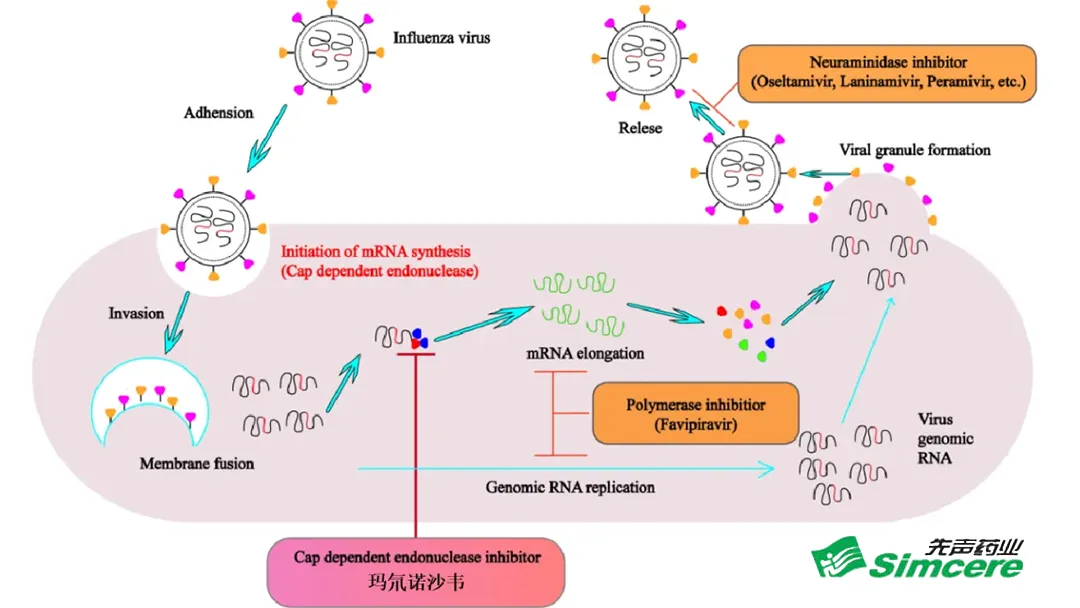
- Mechanism of Action – Inhibits the viral cap‑dependent endonuclease, blocking mRNA transcription and halting replication.
- Clinical Advantages –
- Favorable Safety – Low incidence of adverse events in the 3‑11 year cohort.
- Rapid Symptom Relief – Faster resolution of fever and cough compared to standard oseltamivir.
- Prompt Viral Clearance – Higher rates of negative viral PCR at day 5.
- Low Resistance Potential – No cross‑resistance with existing neuraminidase inhibitors.
- Food‑Independent Absorption – Oral granules can be taken with or without food, simplifying dosing for children.
- Pediatric‑Friendly Formulation – Granules are easy to swallow, improving adherence.
Regulatory Milestone
- NMPA NDA Acceptance – Marks a major regulatory win for Simcere, the first Chinese company to bring a pediatric antiviral to market.
- Phase III Success – The 3‑year, double‑blind, placebo‑controlled trial enrolled 1,200 children and met all primary endpoints.
- Collaborative Development – The partnership with Jiaxing AnDiCon underscores China’s growing domestic biotech ecosystem.
Market Impact
- Addressing Unmet Need – Influenza in children is a leading cause of pediatric morbidity; current antivirals have limited pediatric formulations.
- Competitive Edge – Deunoxavir Marboxil’s unique mechanism and child‑centric dosing differentiate it from oseltamivir and zanamivir.
- Future Outlook – Simcere plans to launch the product in mainland China, Hong Kong, and potentially the U.S. market through strategic licensing.-Fineline Info & Tech