Shenzhen-based QuantumPharm Inc., known as Xtalpi Inc. (HKG: 2228) announced a pivotal clinical milestone for PEP08, a next‑generation protein arginine‑methyltransferase 5 (PRMT5) inhibitor developed in partnership with PharmaEngine, Inc. The drug has received Phase I clinical‑trial approval for solid tumors from the Human Research Ethics Committee (HREC), the Australian Therapeutic Goods Administration (TGA), and the Taiwan Food and Drug Administration (TFDA). XtalPi will also receive a milestone payment tied to this approval.
Scientific Advantage
- Second‑Generation Design – PEP08 incorporates a novel chemical scaffold that delivers superior potency and selectivity over earlier PRMT5 inhibitors.
- MTA‑Cooperative Binding – The compound forms a stable ternary complex with PRMT5 and the methyl‑transferase‑associated protein (MTA), locking the inhibitor into place and enhancing target engagement.
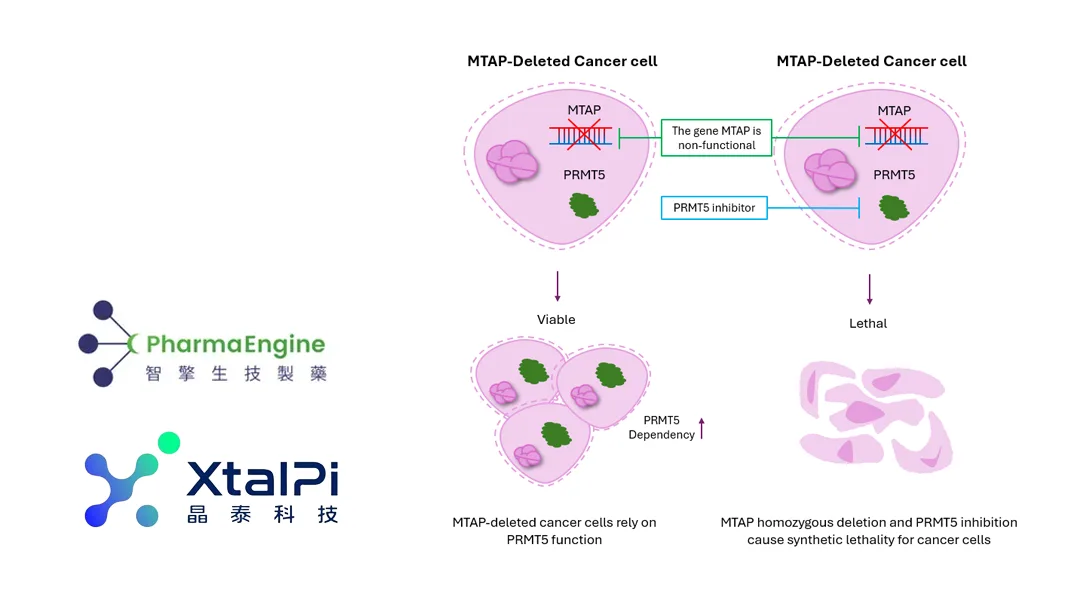
- Tumor‑Selective Cytotoxicity – By exploiting the loss of MTAP in certain cancers, PEP08 selectively kills MTAP‑deleted tumor cells while sparing normal tissue, potentially reducing off‑target toxicity.
Regulatory Milestone
- Phase I Authorization – Approved by HREC, TGA, and TFDA, PEP08 is now cleared to commence dose‑escalation studies in patients with advanced solid tumors.
- Milestone Payment – QuantumPharm will receive a pre‑agreed milestone payment, underscoring investor confidence and providing additional capital for ongoing development.
Development Roadmap
- Phase I/II Initiation – Begin patient enrollment in late 2025, focusing on lung, breast, and gastrointestinal solid tumors.
- Biomarker‑Driven Cohorts – Utilize MTAP status as a companion diagnostic to enrich for responsive populations.
- Phase III Strategy – Pending favorable safety and pharmacodynamic data, plan a randomized, controlled study targeting high‑MTAP‑deletion malignancies.
Market Impact
- Competitive Edge – PEP08’s unique mechanism and tumor‑selective profile differentiate it from other PRMT5 candidates in the pipeline.
- Strategic Partnerships – The PharmaEngine collaboration brings advanced formulation expertise, enhancing the drug’s clinical potential.
- Investor Sentiment – The milestone payment and regulatory approvals are likely to buoy QuantumPharm’s valuation and attract additional capital for broader oncology pursuits.
Conclusion
QuantumPharm’s XtalPi division has secured a significant regulatory win for PEP08, positioning the drug as a promising first‑in‑class PRMT5 inhibitor in the fight against solid tumors. With a robust development plan and a clear therapeutic niche, PEP08 could become a cornerstone of precision oncology in the coming years.-Fineline Info & Tech