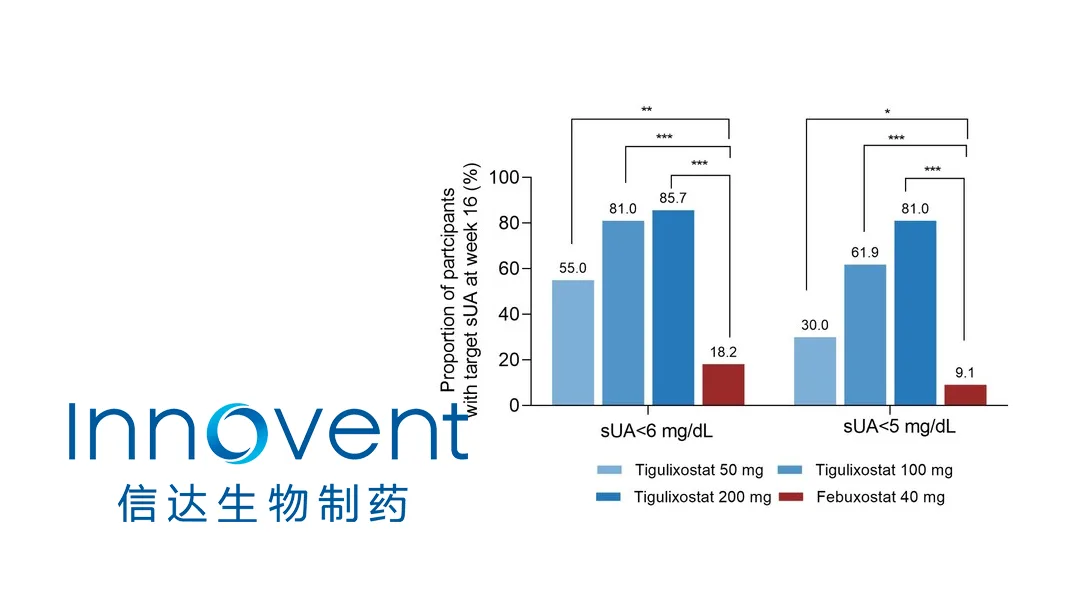
China-based Innovent Biologics Inc. (HKG: 1801) revealed that its next‑generation xanthine oxidase inhibitor (XOI), tigulixostat tablets (IBI128), achieved superior uric‑acid‑lowering efficacy and a favorable safety profile versus febuxostat in a Phase II study presented at the 2025 Asia‑Pacific League of Associations for Rheumatology (APLAR) Congress.
Key Findings
- Efficacy – All dose cohorts of tigulixostat produced a significantly greater reduction in serum uric acid (sUA) compared with the febuxostat control.
- Safety – No dose‑dependent adverse events were observed; the safety profile matched that of febuxostat.
- Clinical Impact – Results support a dose‑dependent increase in sUA target rate, positioning tigulixostat as a promising first‑line therapy for hyperuricemia.
Next Steps
- Phase III Launch – Innovent plans to initiate a registrational Phase III trial of IBI128 in China during the second half of 2025.
- Strategic Collaboration – Since December 2022, Innovent has secured exclusive development and commercialization rights for tigulixostat in China through a partnership with LG Chem.
Development Milestones
| Phase | Outcome | Status |
|---|---|---|
| Phase I | Safety & PK in healthy volunteers | Completed |
| Phase II (China) | Efficacy vs. febuxostat | Completed |
| Phase II (US) | Efficacy vs. febuxostat | Completed |
| Phase III (International) | Registrational data | Completed |
| Phase III (China) | Upcoming | Planned (H2 2025) |
Market Context
- Global XOI Landscape – Current XOI options (e.g., allopurinol, febuxostat) face adherence and tolerability challenges.
- Tigulixostat Advantage – Non‑purine, selective XOI design reduces off‑target effects, potentially improving patient compliance.-Fineline Info & Tech