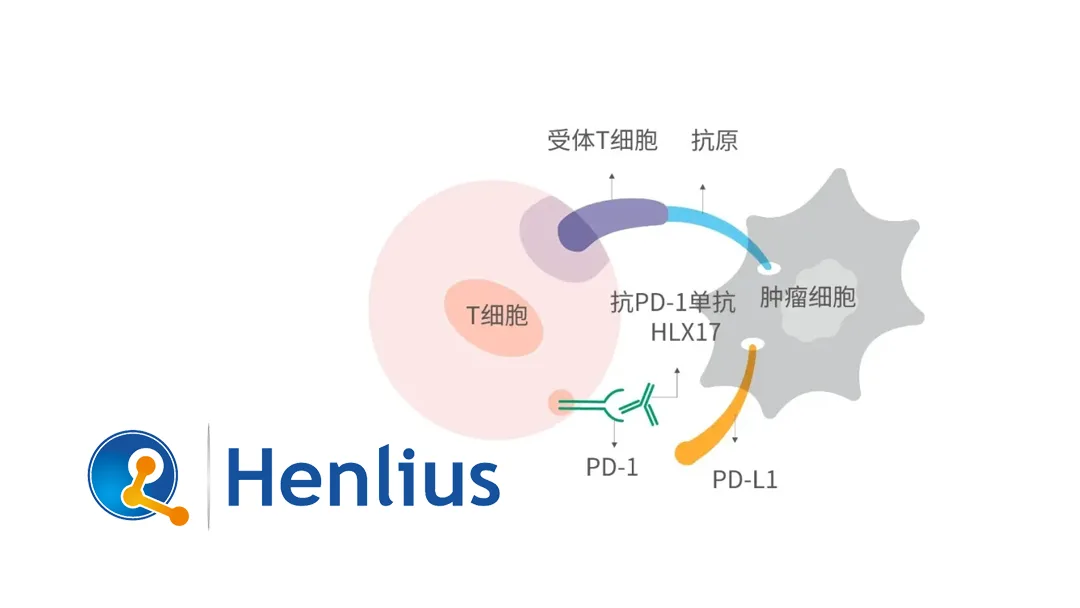
Shanghai Henlius Biotech, Inc. (HKG: 2696) announced that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for HLX17 – a recombinant humanized anti‑PD‑1 monoclonal antibody injection proposed as a pembrolizumab biosimilar. The new IND authorizes clinical testing of HLX17 as an adjuvant therapy for certain resected solid tumors.
What Makes HLX17 a Strong Biosimilar Candidate
- Independent Development – Henlius engineered HLX17 in full compliance with the International Conference on Harmonisation (ICH) guidelines and the regulatory frameworks of the NMPA, EMA, and FDA.
- Comprehensive Comparative Studies –
- Pharmacologic – Demonstrated near‑identical binding affinity and functional potency to reference pembrolizumab.
- Pre‑clinical Pharmacology & Pharmacodynamics – Showed comparable tumor‑infiltrating lymphocyte activation and cytokine release profiles.
- Pharmacokinetics & Immunogenicity – Identical half‑life, clearance rates, and low immunogenicity risk.
These data collectively establish biosimilarity to the reference product, meeting the stringent FDA biosimilar standards.
Clinical Implications
- Adjuvant Use – HLX17 is poised to address a growing market need for effective, cost‑competitive immunotherapies in the adjuvant setting for resected cancers (e.g., colorectal, lung, breast).
- Market Positioning – By offering a biosimilar that matches the safety and efficacy profile of pembrolizumab, Henlius could significantly expand access to anti‑PD‑1 therapy in emerging markets with limited biosimilar options.
Next Steps
- Phase 1/2 Clinical Trials – Begin enrollment in the United States and select international sites to evaluate safety, tolerability, and pharmacokinetics in the adjuvant setting.
- Regulatory Strategy – Leverage the IND approval to expedite a Biologics License Application (BLA) pipeline under the FDA’s Biosimilars pathway.
- Partnership Exploration – Henlius is actively seeking collaborations with large specialty pharmaceutical companies to accelerate global commercialization.
Market Context
The global biosimilars market is projected to surpass USD 25 bn by 2030, driven by cost‑pressure and expanding reimbursement policies. Pembrolizumab, a blockbuster checkpoint inhibitor, represents a key target for biosimilar entry, offering significant opportunities for market penetration and revenue growth.-Fineline Info & Tech