China‑based Sihuan Pharmaceutical Holdings Group Ltd. (HKG: 0460) announced that the Investigational New Drug (IND) for P052 injection, a novel Glucagon‑Like Peptide‑1 (GLP‑1)/Glucagon Receptor (GCGR) dual‑target agonist developed by its subsidiary Huisheng Biopharmaceutical Co., Ltd., has received approval from the National Medical Products Administration (NMPA) for the treatment of type 2 diabetes, overweight and obesity.
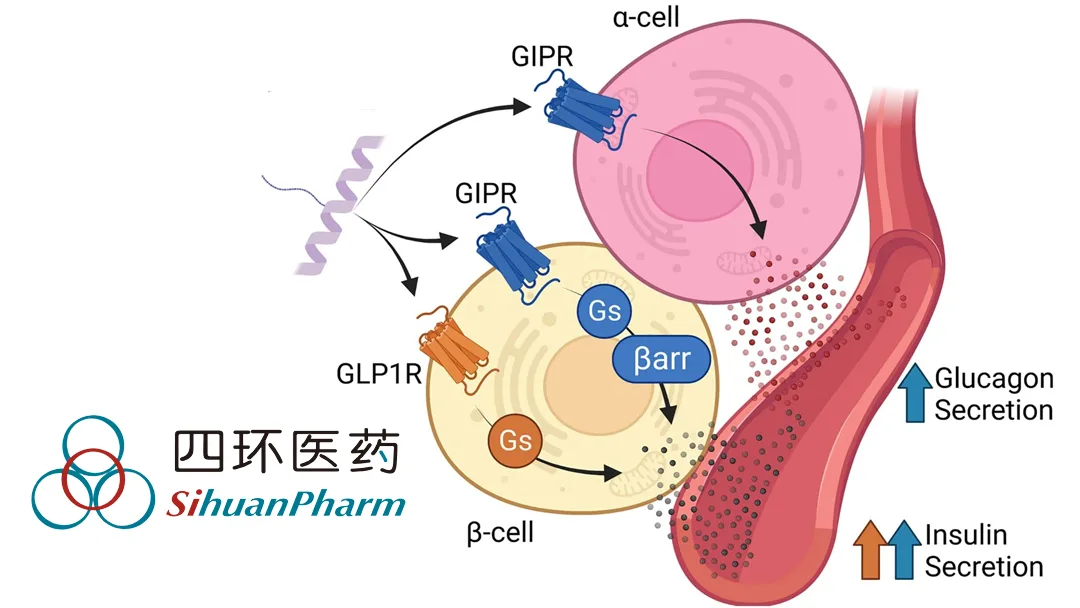
How P052 Works
- GLP‑1 Activation – Stimulates GLP‑1 receptors to boost insulin secretion, lower blood glucose, and induce weight loss.
- GCGR Stimulation – Increases energy expenditure and enhances weight‑loss efficacy while improving hepatic fat metabolism.
- Dual‑Mechanism Advantage – Combines the glycaemic control of GLP‑1 agonists with the metabolic benefits of GCGR activation.
Pre‑Clinical Performance
| Metric | P052 | Semaglutide (GLP‑1 only) |
|---|---|---|
| Hypoglycaemic effect | Comparable | Comparable |
| Weight‑loss effect | Significantly superior | Baseline |
Pre‑clinical studies show that P052 delivers a hypoglycaemic profile on par with the leading GLP‑1 therapy semaglutide, while achieving markedly greater weight loss and improved liver fat metabolism.
Strategic Implications
- First‑Mover Edge – P052 represents the first dual‑target GLP‑1/GCGR therapeutic approved in China, positioning Sihuan Pharma as a pioneer in next‑generation metabolic medicines.
- Portfolio Expansion – The IND paves the way for Phase I/II clinical trials, potentially broadening the company’s diabetes and obesity product line.
- Investor Interest – The approval is expected to boost confidence among shareholders and attract further capital for clinical development.
Next Steps
- Initiate Phase I Trials – Begin safety and dose‑finding studies in healthy volunteers and patients with type 2 diabetes.
- Biomarker Development – Validate GLP‑1 and GCGR engagement markers to guide patient selection.
- Global Regulatory Strategy – Leverage early Chinese approval to support submissions to the FDA and EMA once clinical data mature.-Fineline Info & Tech