China‑listed Kexing Biopharm Co., Ltd. (SHA: 688136) announced today a strategic commercialization agreement with Humanwell PuraCap Pharmaceutical (Wuhan) Co., Ltd., a subsidiary of Humanwell Healthcare Group Co., Ltd. (SHA: 600079). The deal grants Kexing the rights to market nintedanib ethanesulfonate soft capsules worldwide—excluding China (mainland, Hong Kong, Macau, Taiwan), the United States, Saudi Arabia, Ecuador, Bolivia, Paraguay, and Uruguay.
What the Agreement Covers
| Region | Status |
|---|---|
| All other global markets | Kexing holds exclusive commercial rights |
| China (incl. HK, Macau, TW) | Humanwell PuraCap retains sales |
| United States, Saudi Arabia, Ecuador, Bolivia, Paraguay, Uruguay | Excluded from Kexing’s portfolio |
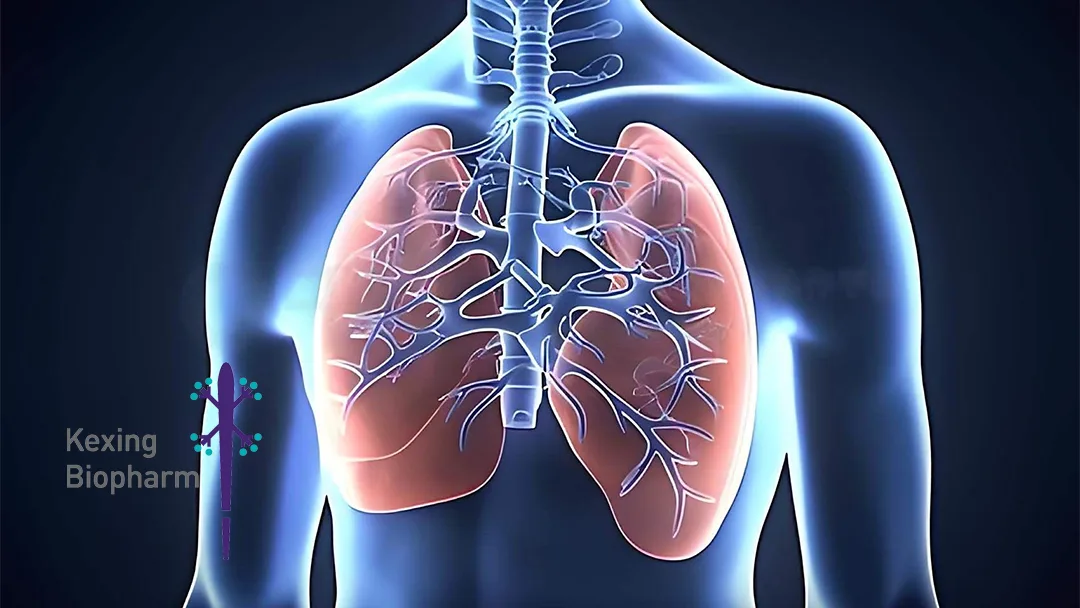
Why Nintedanib Matters
- Mechanism – Multi‑target tyrosine‑kinase inhibitor that blocks VEGFR, PDGFR, and FGFR, curbing fibroblast proliferation and inflammatory pathways.
- Clinical Use – First‑line therapy for idiopathic pulmonary fibrosis (IPF).
- History – Originally launched by Boehringer Ingelheim under the brand Ofev (2017); Humanwell PuraCap’s generic received Chinese approval in September 2023.
Strategic Implications
- For Kexing – Expands its oncology‑dermal portfolio into a high‑growth respiratory indication with proven global demand.
- For Humanwell PuraCap – Secures a dedicated partner to drive commercialization outside its core markets while retaining China‑centric sales.
- For Investors – The partnership leverages Kexing’s robust commercial network and Humanwell’s manufacturing excellence, positioning both companies for accelerated revenue growth in an increasingly competitive IPF landscape.-Fineline Info & Tech