Shanghai‑based FibroX Therapeutics announced that its investigational new drug (IND) application for FibroCell, an allogeneic human fibroblast injection, has received approval from the China Food and Drug Administration (CFDA) for the treatment of moderate‑to‑severe lumbar disc degenerative disease.
What FibroCell Brings to the Back‑Pain Market
- First‑in‑class cell‑based therapy that converts degenerated nucleus pulposus cells into fibrous cells, directly addressing the root cause of disc degeneration.
- Clinical advantage for patients in early‑to‑mid stages of disc degeneration, where current surgical or pharmacologic options are limited.
- Dual mechanism of action:
- Anti‑inflammatory: Suppresses local inflammation within the disc.
- Mechanical reinforcement: Enhances disc stiffness through fibrosis, delaying disease progression.
Delivery and Safety
- Administered via a minimally invasive injection into the annulus fibrosus, reducing the risk of trauma and post‑procedural complications.
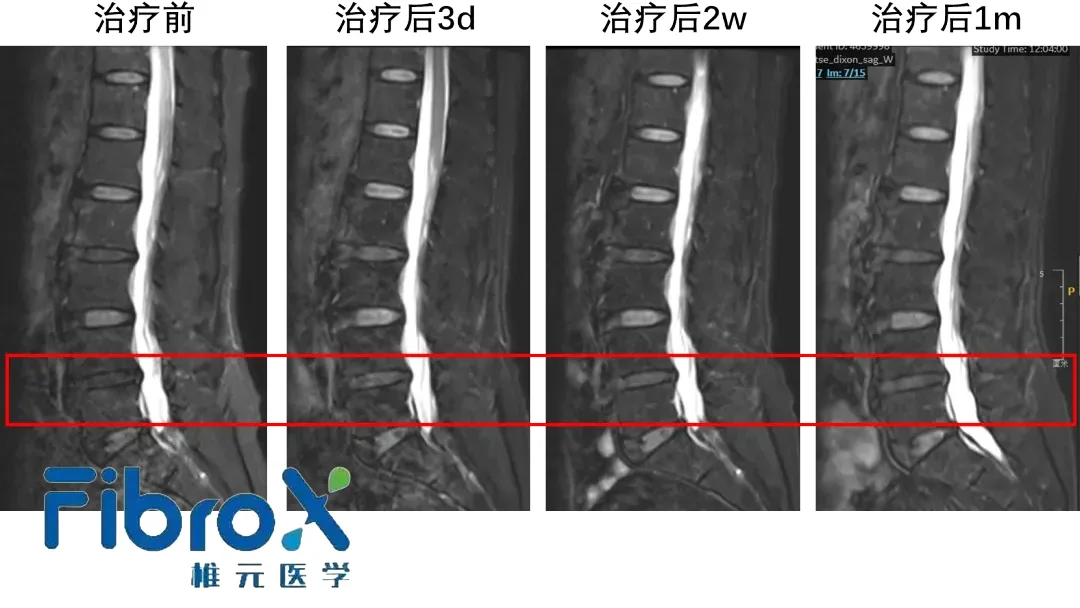
- Early‑stage clinical data show rapid restoration of disc structure and persistent relief of lower‑back pain.
Regulatory and Commercial Outlook
- IND approval paves the way for Phase I/II clinical trials to confirm safety and efficacy in a larger cohort.
- If successful, FibroCell could become the first FDA‑qualified cell therapy for lumbar disc degeneration, opening a multi‑billion‑dollar market for regenerative back‑pain solutions.-Fineline Info & Tech