Ascletis Pharma Inc. (HKG: 1672) announced that its Phase Ib multiple‑ascending‑dose (MAD) study of the oral GLP‑1R agonist ASC30 achieved a 6.5 % mean weight‑loss reduction in obese subjects after 28 days of treatment, as presented at the 61st Annual Meeting of the European Association for the Study of Diabetes (EASD).
Study Design and Objectives
- Randomized, double‑blind, placebo‑controlled trial conducted in the United States.
- Cohorts:
- Cohort 1 – 2 mg, 5 mg, 10 mg, 20 mg weekly dose escalation.
- Cohort 2 – 2 mg, 10 mg, 20 mg, 40 mg weekly dose escalation.
- Target population: obese adults (BMI 30–40 kg/m²).
- Primary endpoints: safety, tolerability, dose‑escalation feasibility, pharmacokinetics (PK), and preliminary efficacy measured by percent body‑weight change.
Efficacy Outcomes
- Cohort 2 (2 mg → 40 mg): 6.5 % placebo‑adjusted weight loss after 28 days.
- Cohort 1 (2 mg → 20 mg): 4.5 % placebo‑adjusted weight loss after 28 days.
- No evidence of a weight‑loss plateau on Day 29, indicating continued efficacy beyond the 28‑day window.
Pharmacokinetics and Dose‑Response
- 20 mg and 40 mg doses achieved superior oral PK at steady state.
- Higher area‑under‑the‑curve (AUC) values correlated positively with greater weight‑loss, supporting a dose‑response relationship.
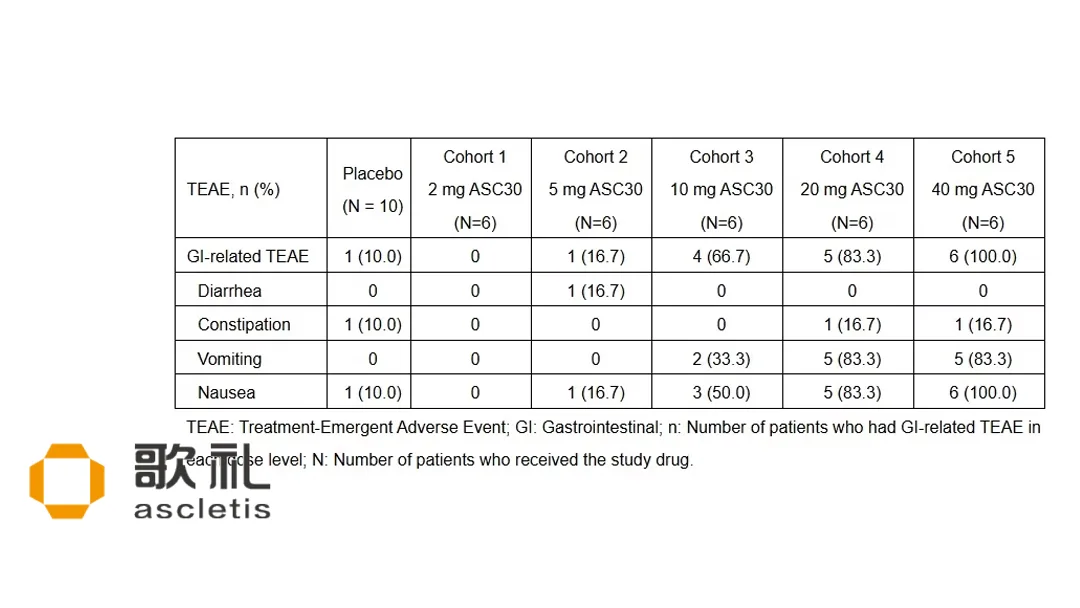
Safety Profile
- No serious adverse events reported in either cohort.
- Tolerability remained consistent across all dose levels, with no dose‑limiting toxicities observed.
Implications and Next Steps
- The data position ASC30 as a promising oral GLP‑1R agonist for obesity management, potentially offering a convenient once‑daily dosing option.
- Ascletis plans to advance ASC30 into Phase II trials to confirm efficacy, refine dosing, and further evaluate long‑term safety.-Fineline Info & Tech