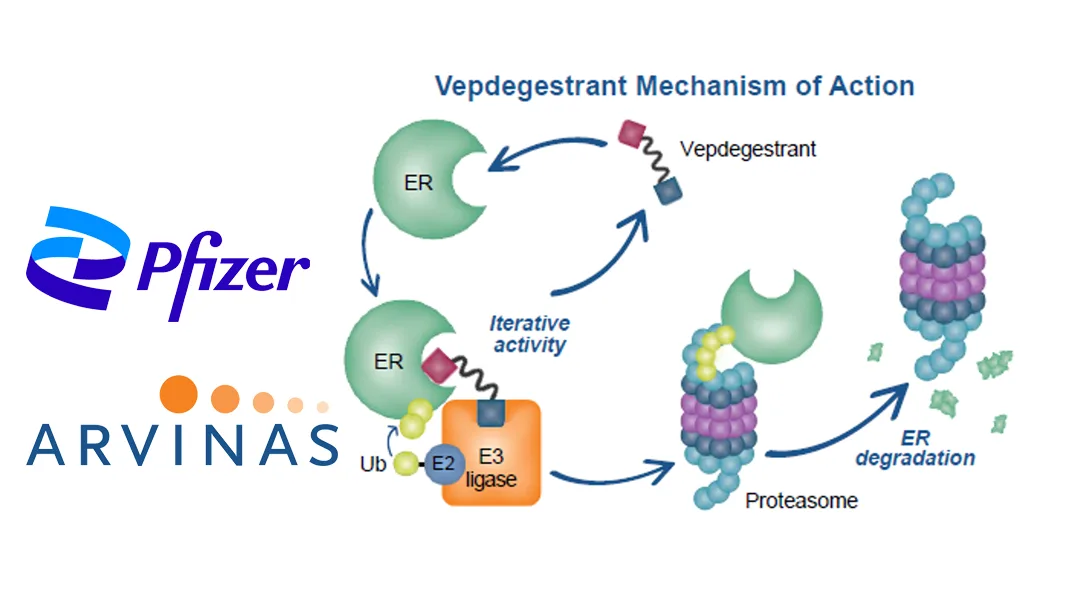
Pfizer Inc. (NYSE: PFE) and Arvinas, Inc. (NASDAQ: ARVN) announced that they are stepping back from the commercialization of the estrogen‑receptor (ER) degrader vepdegestrant. The two companies will hand the primary commercial rights to a third party, a move that follows the termination of two Phase III trials and a shift in strategic focus.
Background
- NDA Filing: On September 17, Pfizer and Arvinas jointly submitted a New Drug Application for vepdegestrant, an oral PROteolysis TArgeting Chimera (PROTAC).
- Prior Development: The program had already scaled back earlier development plans, focusing on vepdegestrant as a second‑line monotherapy after the early‑stage trials.
Clinical Trial Termination
- Phase III End: In May 2025, both the vepdegestrant + Pfizer’s investigational CDK4 inhibitor atirmociclib trial and the vepdegestrant + CDK4/6 inhibitor trial were discontinued.
- Regulatory Insight: Discussions with the FDA suggested that the most promising setting for ER‑targeted therapies is in second‑line and later lines of therapy for patients harboring ESR1 gene mutations.
Strategic Shift
- Focus on Second‑Line Monotherapy: With the NDA now targeting second‑line use, Pfizer and Arvinas decided to outsource commercialization to a partner better positioned to navigate the narrower indication.
- Arvinas Pipeline Rebalancing: The company will re‑allocate resources to its early‑stage PROTAC degraders:
- ARV‑102 – LRRK2 degrader (Phase I)
- ARV‑393 – BCL6 degrader (Phase I)
- ARV‑806 – KRAS G12D degrader (Phase I)
Market Implications
- Investor Outlook: The decision may lower short‑term revenue expectations but frees capital for Arvinas to accelerate its next‑generation degraders.
- Competitive Landscape: Vepdegestrant’s commercial future will now depend on the partner’s ability to secure reimbursement and market access in a highly competitive oncology arena.-Fineline Info & Tech