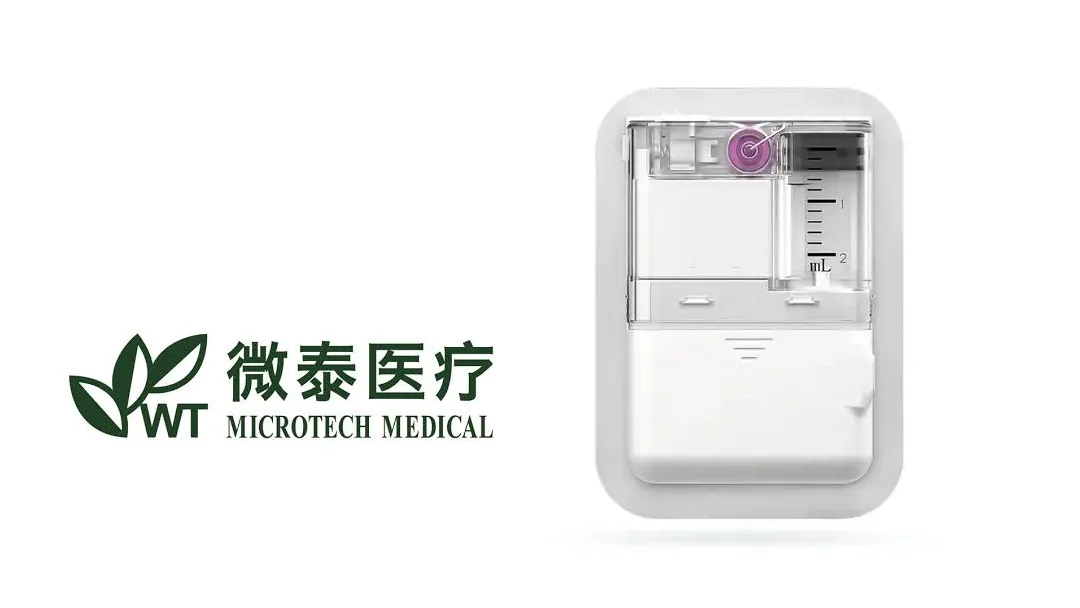
MicroTech Medical (Hangzhou) Co., Ltd. (HKG: 2235) announced that China’s National Medical Products Administration (NMPA) has approved the Equil Patch Insulin Pump for use in diabetic patients aged 3–17 years. The approval expands the product’s indication beyond the adult population, positioning MicroTech as a key player in pediatric diabetes care.
Product Overview
- Patch‑Based Delivery – The Equil Patch delivers insulin via a flexible, adhesive patch that continuously infuses the drug, mimicking the body’s natural insulin secretion.
- Precision & Convenience – With real‑time glucose monitoring and adjustable basal rates, the device offers precise dosing while reducing the need for multiple injections.
- User‑Friendly Design – The patch’s discreet, skin‑compatible material is suitable for children and adolescents, encouraging adherence and improving quality of life.
Regulatory Milestone
- NMPA Approval – The recent clearance covers patients aged 3–17, a demographic for which few closed‑loop systems exist in China.
- Strategic Expansion – The approval follows the 2024 launch of the adult indication, marking a rapid two‑stage rollout within the domestic market.
Market Impact
- Growing Pediatric Diabetes – China’s pediatric diabetes prevalence is rising, creating a sizable unmet need for wearable insulin therapies.
- Competitive Edge – By securing pediatric approval, MicroTech positions itself ahead of competitors that still rely on traditional injections or adult‑only devices.
- Future Outlook – The company plans to pursue additional indications, including type 1 diabetes in adolescents, and to explore international regulatory pathways in the coming year.