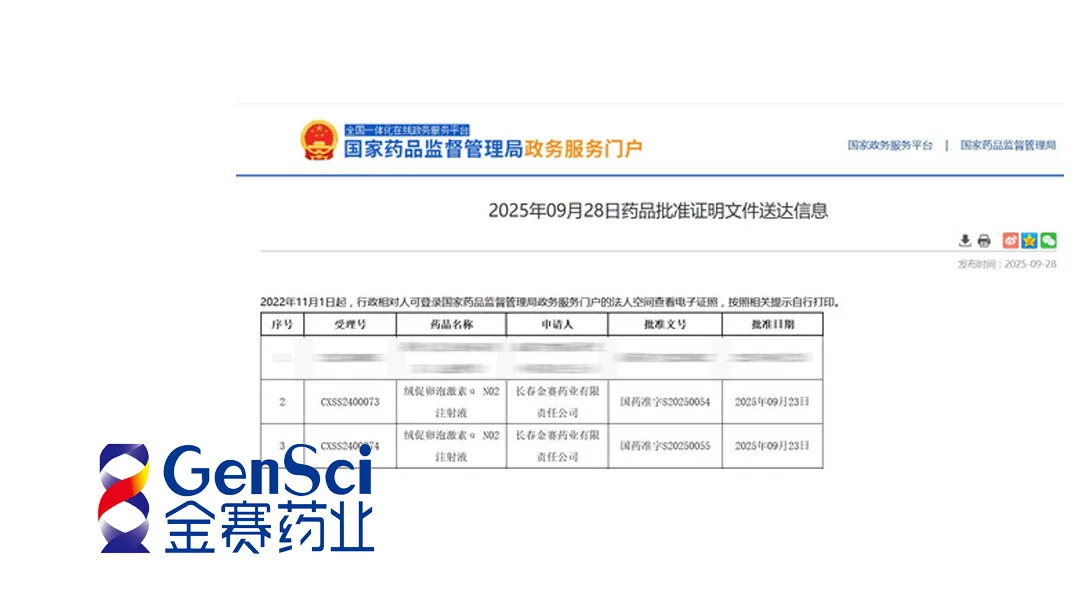
China‑based Changchun GeneScience Pharmaceutical Co. Ltd. announced that its long‑acting recombinant human follicle‑stimulating hormone—CTP fusion protein injection, marketed as Follitropin Alpha N02 Injection, has received official marketing approval in China. The decision follows the company’s successful submission to the National Medical Products Administration (NMPA) and marks a significant advance in assisted‑reproductive‑technology (ART) options for Chinese patients.
Clinical Indication
Follitropin Alpha N02 is approved for use in combination with a gonadotropin‑releasing hormone (GnRH) antagonist during controlled ovarian stimulation (COS). The single subcutaneous injection replaces the traditional seven‑day daily FSH regimen, offering a more convenient and potentially cost‑effective approach to inducing multiple follicle development.
Market Context
The world’s first long‑acting FSH, Elonva, received EU approval in 2010. In China, patients have long been burdened by daily injections, underscoring the critical need for long‑acting stimulation therapies. GeneScience pioneered the country’s first domestically produced recombinant human FSH in 2015, but that formulation remained short‑acting. With infertility prevalence now at 18.2 % in China, demand for streamlined ART solutions is rising sharply.
Strategic Implications
The approval positions Changchun GeneScience as a key player in the rapidly expanding Chinese reproductive‑health market. By delivering a single‑dose, long‑acting FSH product, the company addresses both clinical efficacy and patient adherence, potentially reshaping standard COS protocols nationwide.-Fineline Info & Tech