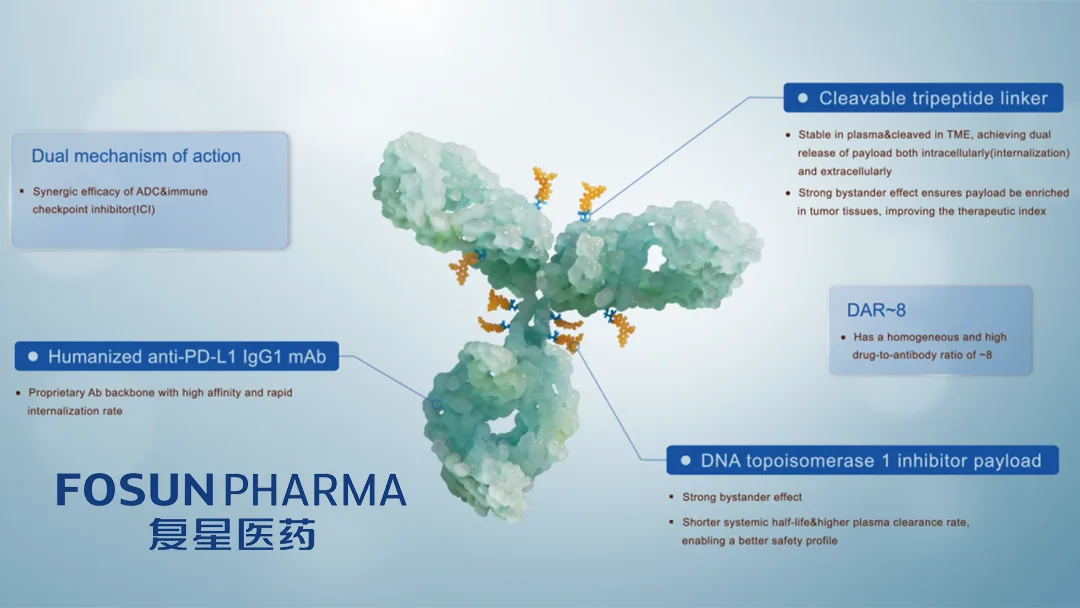
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. (SHA: 600196) announced that the National Medical Products Administration (NMPA) has granted approval for a Phase 1b/2 clinical trial of the HLX43 injection in combination with HLX07 for patients with advanced or metastatic solid tumors.
About the Candidate Drugs
- HLX43 – an antibody‑drug conjugate (ADC) that targets PD‑L1. Previous trials have evaluated the ADC in non‑small cell lung cancer (NSCLC) and thymic carcinoma (TC), demonstrating a favorable safety profile and preliminary activity.
- HLX07 – a recombinant, humanized monoclonal antibody that blocks EGFR. Phase 2 studies have shown activity in NSCLC and advanced cutaneous squamous cell carcinoma (CSCC), with manageable toxicity.
Both agents are classified as Category 1 therapeutic biological products in China.
Significance of the Combination
The HLX43 + HLX07 regimen represents a first‑in‑class dual‑target strategy, simultaneously inhibiting PD‑L1‑mediated immune evasion and EGFR‑driven tumor proliferation. No globally approved combination therapy currently matches this dual‑mechanism approach, positioning the trial as a potential breakthrough in solid‑tumor therapeutics.
Regulatory Context
- NMPA Approval removes a major regulatory barrier, allowing Fosun to initiate internationally coordinated Phase 1b/2 testing.
- The approval aligns with China’s broader initiative to accelerate advanced‑stage oncology trials and expand access to innovative biologics.
Market Implications
- Fosun’s portfolio now includes a novel ADC‑EGFR combination that could capture a significant share of the advanced‑solid‑tumor market if clinical endpoints are met.
- Positive outcomes may prompt global regulatory submissions (e.g., EMA, FDA) and open pathways for public‑private partnerships or licensing deals.-Fineline Info & Tech