China‑based Sichuan Biokin Pharmaceutical Co., Ltd. (SHA: 688506) announced that its wholly‑owned subsidiary SystImmune, Inc. has achieved a pivotal milestone in the global Phase 2/3 IZABRIGHT‑Breast01 trial of the bispecific antibody‑drug conjugate (ADC) iza‑bren (BL‑B01D1). The achievement unlocks the first near‑term contingent payment of USD 250 million under the exclusive license and collaboration agreement with Bristol‑Myers Squibb Company (BMS, NYSE: BMY), with the payment expected in the near future.
Deal Overview
| Element | Details |
|---|---|
| License & Collaboration | Dec 2023 agreement between SystImmune, Inc. (Sichuan Biokin) and BMS |
| Territorial Scope | • SystImmune – exclusive development, commercialization, and production of BL‑B01D1 in mainland China; partial supply for global use. • BMS – exclusive global development and commercialization outside China. |
| Payment Structure | • USD 800 million non‑refundable upfront payment (already received). • Up to USD 250 million near‑term contingent payments (triggered by current milestone). • Potential additional payments up to USD 7.1 billion upon future development, regulatory, and sales milestones. |
Clinical Milestone
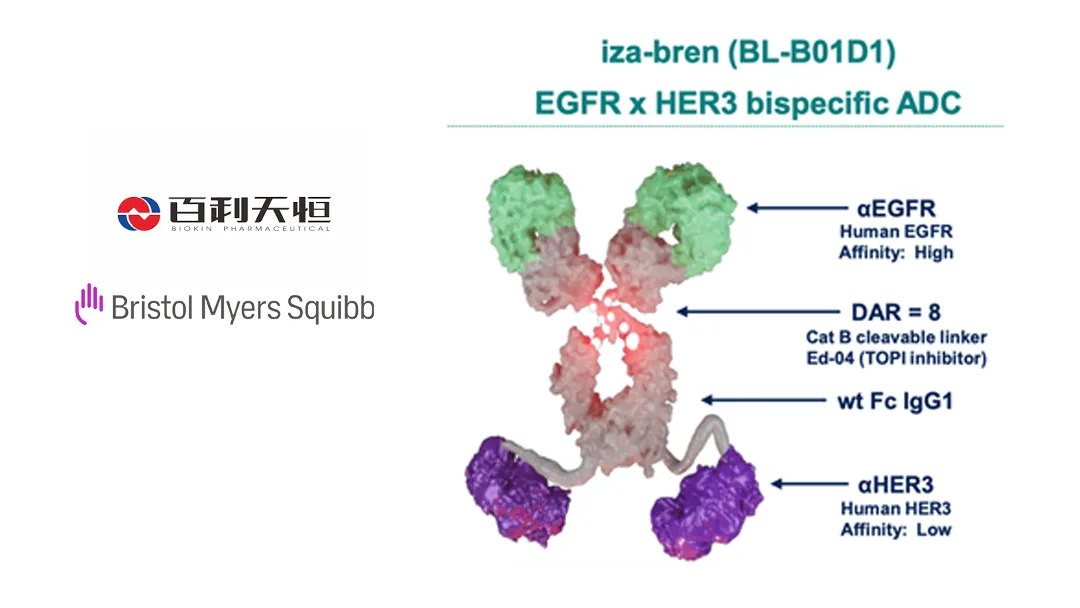
- Drug Profile – iza‑bren (BL‑B01D1) is an EGFR×HER3 bispecific ADC targeting HER3‑positive breast cancers.
- Trial Success – The IZABRIGHT‑Breast01 study has reached a pivotal endpoint in its Phase 2/3 program, validating the clinical advantage of the bispecific ADC in HER3‑driven tumors.
- Implication for Oncology – The milestone underscores the therapeutic potential of bispecific ADCs and positions SystImmune/Biokin as a key partner in BMS’s global oncology pipeline.
Financial Impact
- Immediate Cash Flow – The USD 250 million payment strengthens SystImmune’s balance sheet and supports ongoing R&D activities in China.
- Revenue Upside – With the potential for up to USD 7.1 billion in milestone payments, the agreement offers a scalable revenue engine tied to clinical success and market penetration.
- Investor Confidence – The partnership exemplifies a successful public‑private collaboration, enhancing Biokin’s reputation within the biopharmaceutical ecosystem.
Strategic Outlook
Sichuan Biokin and BMS continue to collaborate on the development, commercialization, and production of BL‑B01D1 worldwide. The partnership leverages Biokin’s manufacturing capabilities in China and BMS’s global commercialization expertise, positioning both companies to capitalize on the growing demand for next‑generation ADC therapies.-Fineline Info & Tech