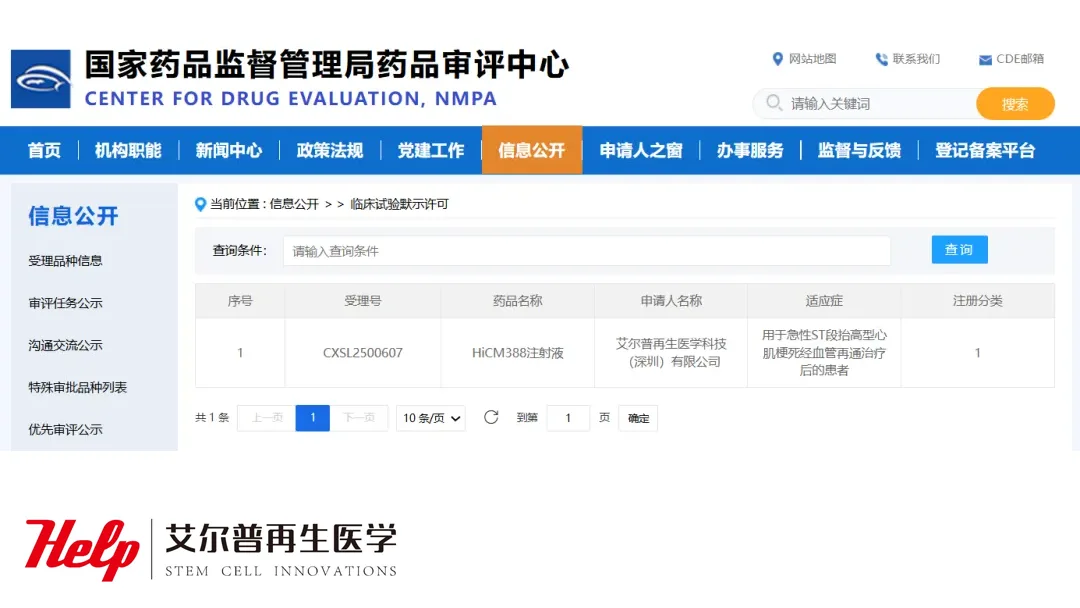
HELP Therapeutics Corp., a pioneer in cell‑based therapeutics, announced that the National Medical Products Administration (NMPA) has formally approved its Investigational New Drug (IND) application for Human Umbilical Cord Mesenchymal Stem Cells for Injection (HiCM388) (Acceptance Number: CXSL2500607). The Class 1 drug targets patients who have undergone revascularization following an acute ST‑segment elevation myocardial infarction (STEMI) and represents the first cell‑based innovative product in China for this indication.
Key Highlights
- NMPA Approval – IND for HiCM388 cleared for clinical testing in China.
- Target Indication – Post‑revascularization care for acute STEMI patients.
- First‑Mover Status – China’s inaugural cell‑based therapy for myocardial infarction.
HELP Therapeutics’ Track Record
| Milestone | Detail |
|---|---|
| Global First | iPSC‑derived cardiomyocyte therapy for severe heart failure received dual IND approval in China and the U.S., launching multi‑center trials in China, the U.S., Singapore, and Thailand. |
| R&D Focus | Long‑standing commitment to innovative cell‑based drugs for unmet clinical needs. |
| Portfolio Expansion | HiCM388 adds a new therapeutic modality to the company’s pipeline of regenerative medicines. |
Clinical Development Path
- Phase I/II – Initial safety and dose‑finding studies in STEMI patients with prior revascularization.
- Phase IIb/III – Efficacy evaluation on cardiac function, infarct size reduction, and long‑term outcomes.
- Regulatory Strategy – Parallel submissions to the U.S. FDA and EU EMA to accelerate global access.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech