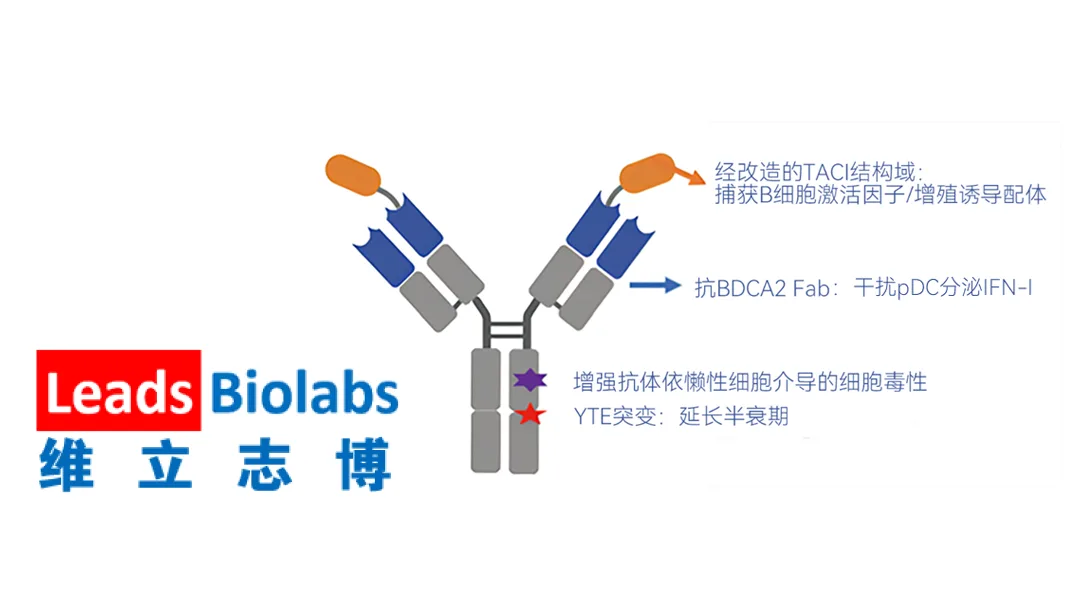
Nanjing Leads Biolabs Co., Ltd. (HKG: 9887) today announced an exclusive global partnership with Dianthus Therapeutics (NASDAQ: DNTH) to jointly advance the novel anti‑BDCA2/TACI bispecific fusion protein LBL‑047 (to be marketed as DNTH212 outside Greater China). The collaboration combines Leads Biolabs’ first‑in‑class, long‑acting bispecific platform with Dianthus’ expertise in global development, manufacturing, and commercialization.
Key Highlights
| Item | Detail |
|---|---|
| Product | LBL‑047 (DNTH212) – anti‑BDCA2/TACI bispecific fusion protein |
| Regulatory Status | U.S. IND clearance granted; IND application accepted in China |
| Strategic Scope | Leads Biolabs retains rights in Greater China; Dianthus holds exclusive rights outside Greater China |
| Financial Terms | Up to USD 38 million in upfront and near‑term milestone payments (USD 20 m upfront, USD 5 m Q4 2025, up to USD 13 m milestone). Potential transaction value up to USD 1 billion |
| Revenue Model | Leads Biolabs eligible for tiered royalties (mid‑single digits to low double digits) on net sales outside Greater China |
About LBL‑047
- Mechanism of Action – Selectively eliminates plasmacytoid dendritic cells (pDCs) to curb Type I interferon (IFN‑I) production while simultaneously inhibiting the BAFF/APRIL signaling pathway to block B‑cell activation, survival, and antibody production.
- First‑In‑Class – Represents a new therapeutic class of long‑acting bispecific fusion proteins targeting BDCA2/TACI.
- Clinical Potential – Holds promise for autoimmune and inflammatory disorders where pDC‑driven IFN‑I and BAFF/APRIL pathways are dysregulated.
Strategic Rationale
- Leads Biolabs – Strengthens its Greater China portfolio and retains control over key intellectual property while monetizing its pre‑clinical asset through a lucrative partnership.
- Dianthus Therapeutics – Gains a high‑potential asset with a clear regulatory pathway, enabling rapid entry into the global therapeutic landscape.
- Patients – The partnership accelerates access to a potentially disease‑modifying therapy for conditions driven by aberrant innate immune activation.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech