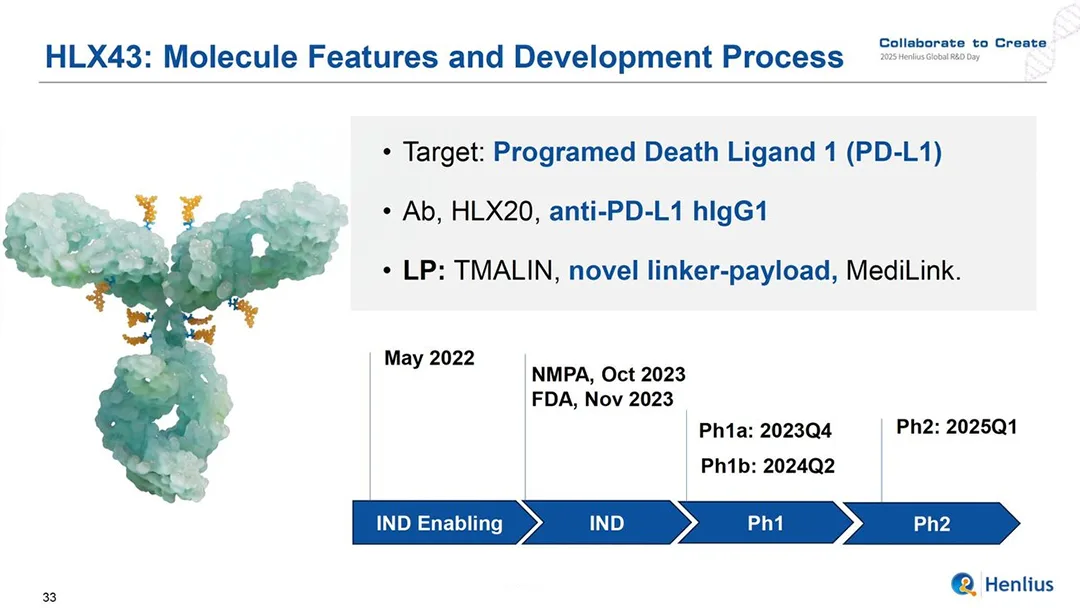
Shanghai Henlius Biotech, Inc. (HKG: 2696) announced today that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to its innovative programmed‑death‑ligand‑1 (PD‑L1)‑targeting antibody‑drug conjugate (ADC), HLX43, for the treatment of thymic epithelial tumors (TETs).
Product Highlights
- Dual‑Mechanism ADC – HLX43 combines immune‑checkpoint blockade with a potent cytotoxic payload, delivering a two‑pronged attack on tumor cells.
- Broad‑Spectrum Activity – In addition to TETs, HLX43 is advancing in non‑small‑cell lung cancer (NSCLC), demonstrating robust efficacy across patient subgroups.
2025 WCLC Data Snapshot
| Cohort | Confirmed ORR (cORR) | Dose (mg/kg) | Safety |
|---|---|---|---|
| EGFR wild‑type NSCLC | 46.7 % | – | Favorable |
| EGFR wild‑type, 2.5 mg/kg | 60.0 % | 2.5 | Favorable |
| PD‑L1 negative (TPS < 1 %) | 38.1 % | – | 85.7 % Disease‑Control Rate (DCR) |
- Key Insight – HLX43 maintains high activity even in PD‑L1‑negative patients, expanding its therapeutic reach beyond traditional biomarkers.
Strategic Implications
- Orphan Designation – The FDA ODD status provides Henlius with market‑exclusivity incentives, expedited review pathways, and potential pricing advantages for TET patients.
- Pipeline Expansion – HLX43’s promising data in NSCLC support parallel development tracks, positioning Henlius as a leader in next‑generation ADCs for solid tumors.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech