Summit Therapeutics, the former China partner of Akeso Inc. (HKG: 9926), announced today that it will file a Biologics License Application (BLA) with the U.S. Food and Drug Administration (FDA) in Q4 2025. The application seeks approval for ivonescimab (AK112) in combination with standard chemotherapy for patients with EGFR‑mutated, non‑squamous non‑small‑cell lung cancer (NSCLC) that has progressed after third‑generation EGFR‑tyrosine kinase inhibitor (TKI) therapy.
Product Overview
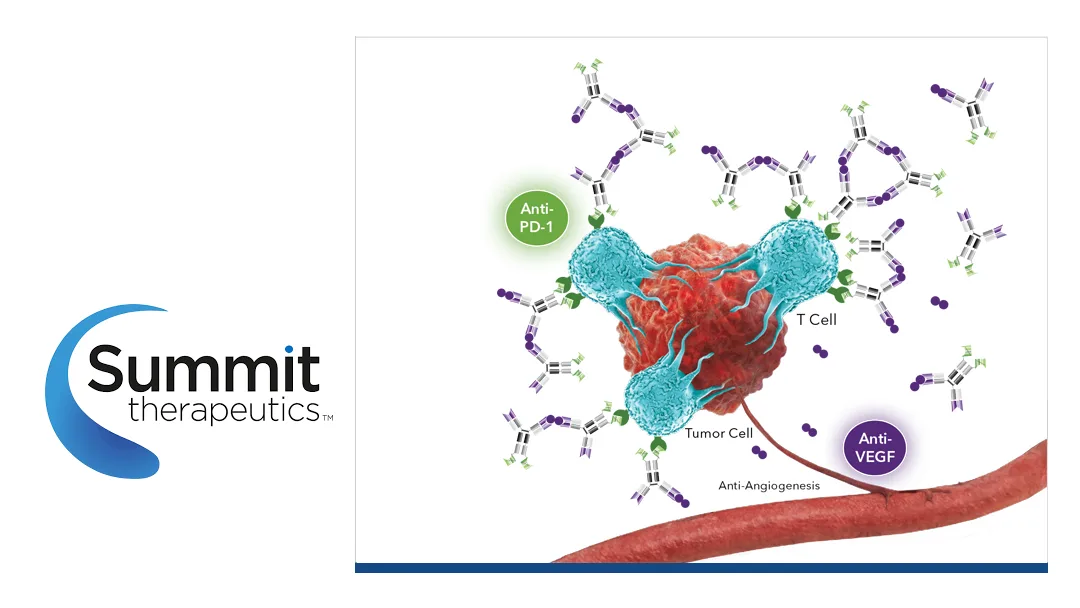
- Ivonescimab (AK112) – a first‑in‑class PD‑1/VEGF bispecific antibody that merges immuno‑oncology with anti‑angiogenesis.
- Established Clinical Success – Approved by China’s National Medical Products Administration (NMPA) in May 2024 for locally advanced or metastatic non‑squamous NSCLC post‑EGFR‑TKI.
- Market Impact – Became the world’s first bispecific antibody approved for oncology, and was added to China’s National Reimbursement Drug List (NRDL) in November 2024.
Strategic Milestone
- U.S. Expansion – Summit’s Q4 2025 BLA submission will extend ivonescimab’s reach to the largest oncology market, targeting a subset of NSCLC patients with limited options after TKI failure.
- Combination Therapy – The proposed regimen pairs ivonescimab with chemotherapy, leveraging synergistic mechanisms to enhance tumor control.
- Regulatory Pathway – Summit’s prior U.S. licensing of ex‑China rights (December 2022) positions it to navigate FDA review efficiently.
Company Context
- Summit Therapeutics – Focused on bringing innovative oncology therapies to the U.S., leveraging strategic partnerships.
- Akeso Inc. – Continues to develop and commercialize ivonescimab worldwide, with Summit handling U.S. launch and commercialization.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech