China‑based Harbour BioMed (HKG: 2142) announced positive results from its multicenter, open‑label Phase II trial (NCT 05167071) evaluating porustobart (HBM4003), a next‑generation, fully human heavy‑chain‑only anti‑CTLA‑4 antibody, in combination with tislelizumab for microsatellite stable (MSS) metastatic colorectal cancer (mCRC).
Key Takeaways
| Metric | Result |
|---|---|
| Objective Response Rate (ORR) | 34.8 % (8/23 partial responses) |
| Disease Control Rate (DCR) | 60.9 % (8 partial + 6 stable) |
| Median Progression‑Free Survival (mPFS) | 4.2 months |
| Safety | No Grade 4 or fatal TEAEs; manageable profile |
Trial Design
- Population: 24 patients with non‑liver metastatic MSS mCRC, all having received ≥ 2 prior lines of therapy; 66.7 % had lung metastases.
- Treatment Regimen: HBM4003 0.3 mg/kg + tislelizumab 200 mg every 21 days.
- Primary Endpoint: ORR per RECIST 1.1.
Efficacy Highlights
| Endpoint | Value | Interpretation |
|---|---|---|
| ORR | 34.8 % (8/23) | First‑in‑class anti‑CTLA‑4/PD‑1 combo shows clinically meaningful tumor shrinkage in a refractory MSS mCRC cohort. |
| DCR | 60.9 % (14/23) | Majority of patients experienced disease stabilization or response. |
| mPFS | 4.2 months | Extends time to progression relative to historical data for this population. |
Safety Profile
- Treatment‑emergent AEs: 100 % experienced ≥ 1 TEAE; most were Grade 1‑2.
- Grade ≥ 3 TEAEs: 4 patients (17 %) – all manageable.
- Grade 4 or fatal TEAEs: None reported.
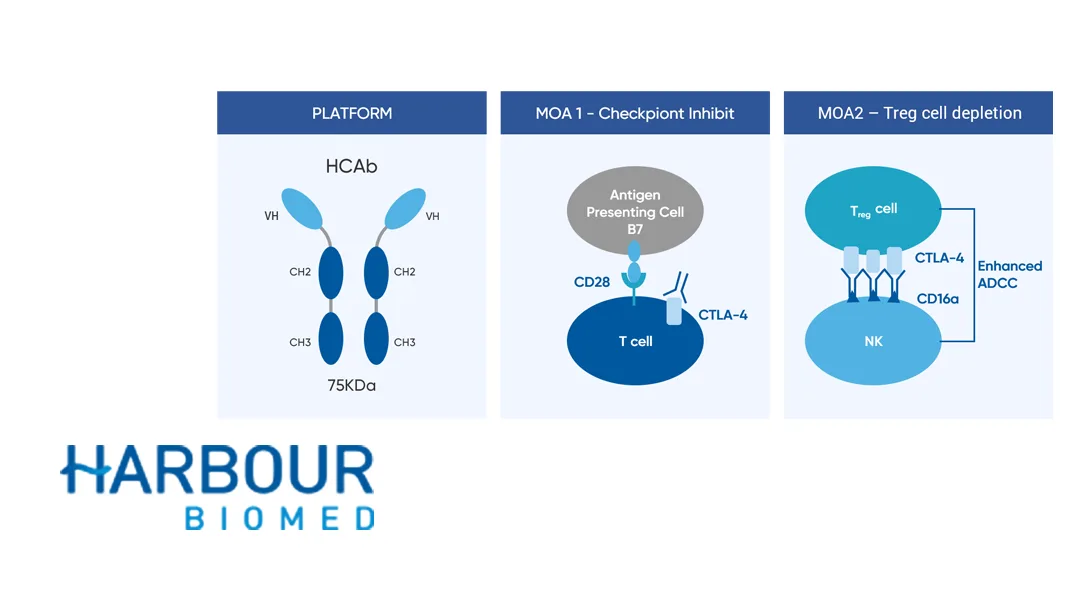
Porustobart: A First‑of‑Its‑Kind
- Platform: HCAb Harbour Mice, producing fully human heavy‑chain‑only antibodies.
- Key Advantages: Enhanced Treg depletion, optimized PK, and an improved safety profile versus conventional anti‑CTLA‑4 agents.
- Regulatory Status: First fully human heavy‑chain‑only antibody to enter global clinical development.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech