China‑based Alphamab Oncology (HKG: 9966) announced today that the U.S. Food and Drug Administration (FDA) has granted Fast‑Track Designation (FTD) to its independently developed HER2 bispecific antibody‑drug conjugate (ADC) JSKN003. The designation targets patients with advanced or metastatic platinum‑resistant recurrent epithelial ovarian, primary peritoneal, or fallopian‑tube cancer (PROC), irrespective of HER2 expression level.
Product Highlights
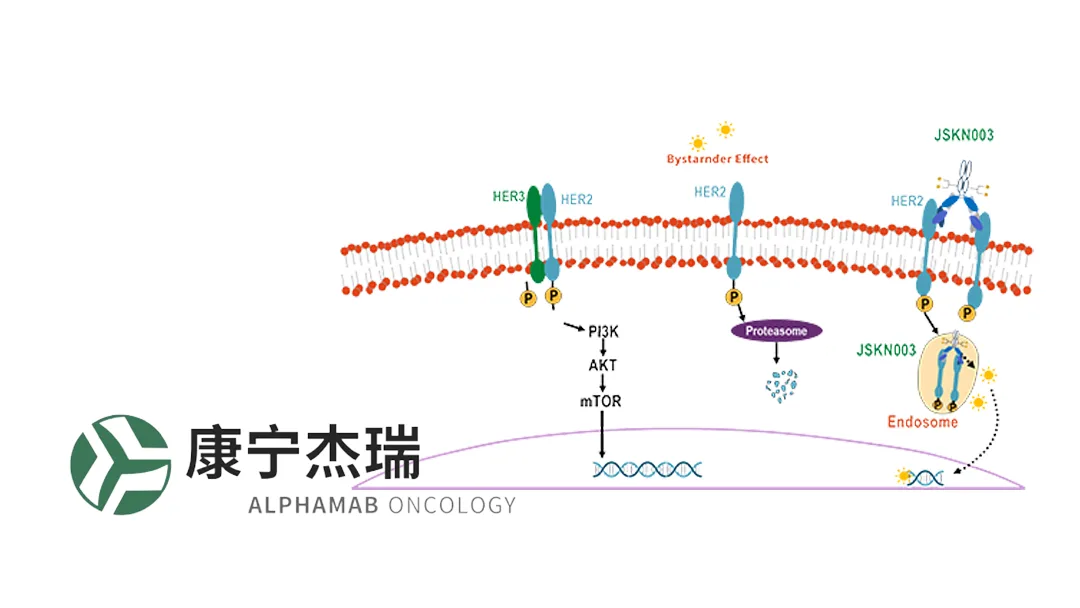
- Homogeneous, DAR‑4 ADC – JSKN003 is engineered via site‑specific conjugation to Fc glycans of anbenitamab, achieving a drug‑to‑antibody ratio of 4.
- Dual‑epitope Binding – The bispecific format simultaneously engages two HER2 epitopes, enhancing cellular endocytosis and intracellular release of a topoisomerase‑I inhibitor.
- Superior Pharmacology – Compared with existing ADCs, JSKN003 shows improved serum stability, lower hematologic toxicity, stronger tumor inhibition, and a pronounced bystander killing effect, widening its therapeutic window.
Strategic Partnership in China
In September 2024, Alphamab entered a licensing agreement with JMT‑Bio, a wholly‑owned subsidiary of CSPC Pharmaceutical Group. JMT‑Bio now holds the exclusive license to develop, commercialise, and sublicense JSKN003 for all oncology indications in Mainland China (excluding Hong Kong, Macau, and Taiwan) and serves as the sole Marketing Authorization Holder (MAH) for the region. Alphamab retains exclusive manufacturing rights.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech