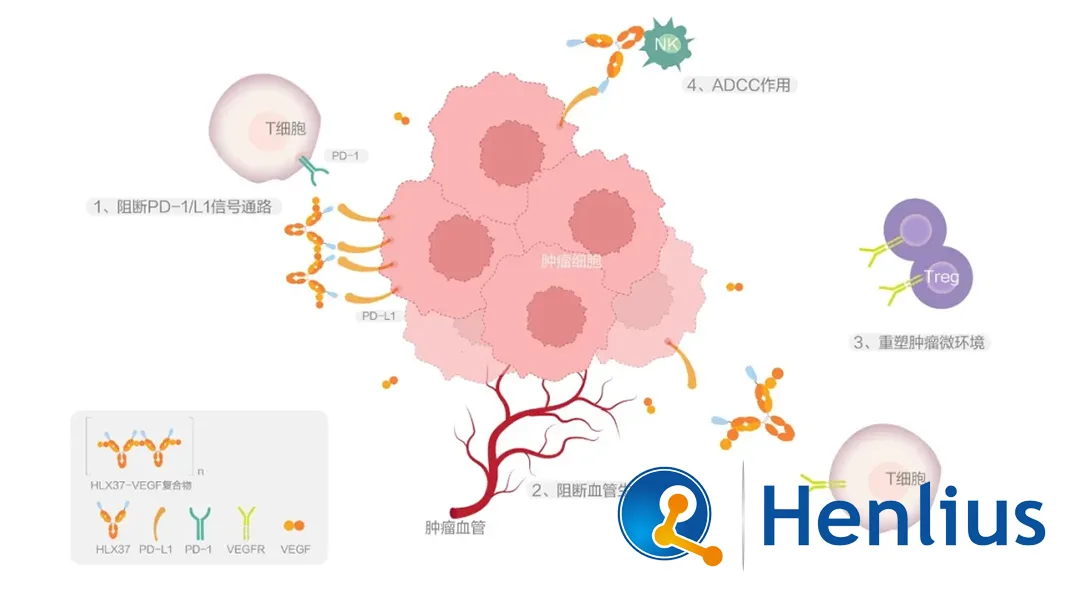
Shanghai Henlius Biotech, Inc. (HKG: 2696) announced that its self-developed investigational new drug, HLX37 (Recombinant Humanized Anti-PD-L1 and Anti-VEGF Bispecific Antibody), has received approval from the China National Medical Products Administration (NMPA) to conduct a Phase I clinical study for the treatment of advanced/metastatic solid tumors.
Regulatory Milestone & Drug Profile
| Attribute | Details |
|---|---|
| Company | Shanghai Henlius Biotech, Inc. (2696.HK) |
| Product | HLX37 |
| Drug Class | Recombinant humanized anti-PD-L1 and anti-VEGF bispecific antibody |
| Approval | NMPA Phase I clinical study IND approval |
| Indication | Advanced/metastatic solid tumors |
| Innovation | Dual-target design targeting both immunosuppression and angiogenesis |
Mechanism of Action & Differentiation
HLX37 combines two therapeutic pathways in a single bispecific antibody:
- PD-L1 Blockade: Blocks binding of PD-L1 on tumor cells to PD-1 on immune cells (e.g., T-cells), releasing tumor immunosuppression and restoring T-cell cytotoxicity
- VEGF Inhibition: Targets VEGF to reduce tumor angiogenesis, limiting blood supply for tumor growth and metastasis
- Synergistic Design: Dual-target approach expected to generate synergistic anti-tumor effects and potentially reduce drug resistance
- Tumor Microenvironment Enrichment: Specific binding to PD-L1 on tumor cells facilitates enrichment of the anti-VEGF functional domain within the tumor microenvironment
- Competitive Advantage: Aims to achieve greater therapeutic effect than the combination of separate anti-PD-L1 and anti-VEGF monoclonal antibodies
Clinical Development Path
| Phase | Study Design | Timeline |
|---|---|---|
| Phase I | Safety, tolerability, and preliminary efficacy in advanced/metastatic solid tumors | Initiation Q1 2026 |
| Next Steps | Dose escalation and expansion cohorts; potential combination studies | 2026-2027 |
Market Context & Strategic Outlook
- Solid Tumor Market: China has >4 million new solid tumor cases annually; targeted therapy market exceeds $15 billion
- Bispecific Antibody Trend: Dual-target biologics gaining traction as next-generation cancer immunotherapies
- Competitive Landscape: HLX37 enters a crowded but growing market; differentiation through co-localization mechanism
- Pipeline Synergy: Strengthens Henlius’ oncology portfolio alongside its established PD-1 inhibitor portfolio
- Global Potential: China approval pathway may support future US/EU development strategies
Forward‑Looking Statements
This brief contains forward‑looking statements regarding HLX37 development timelines, clinical outcomes, and market potential. Actual results may differ due to clinical, regulatory, and competitive uncertainties.-Fineline Info & Tech