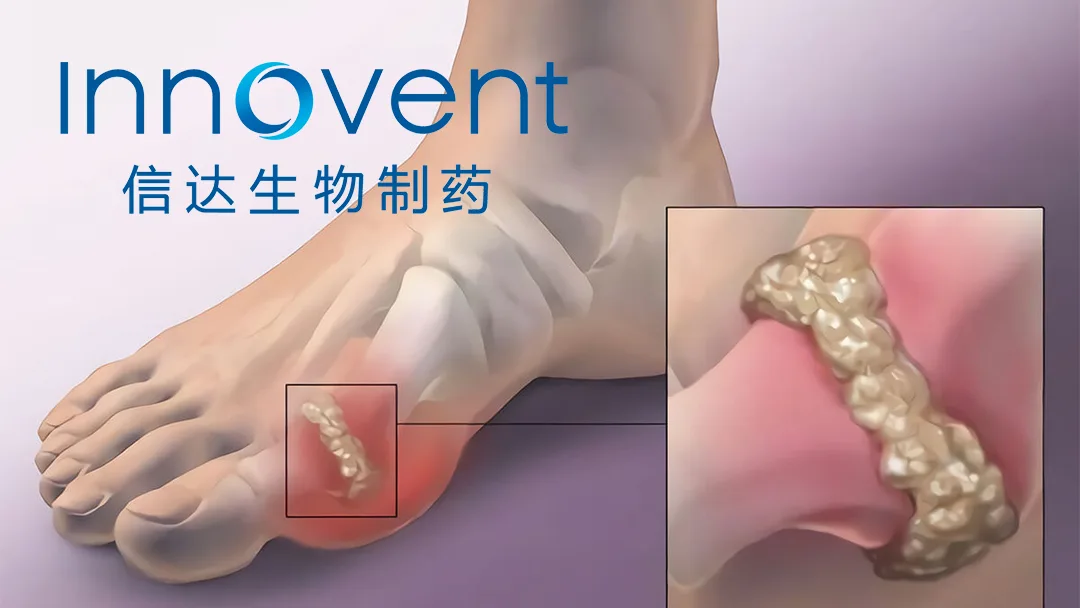
Innovent Biologics, Inc. (HKG: 1801) announced that the first participant has been dosed in a Phase 1 single‑ascending‑dose (SAD) trial of IBI3011, an anti‑IL‑1RAP monoclonal antibody targeting inflammatory and autoimmune diseases. The study will enroll 40 healthy volunteers and 24 acute gout patients to evaluate safety, tolerability, and pharmacokinetics.
Study Design & Regulatory Path
| Item | Details |
|---|---|
| Trial Identifier | NCT06754321 (China) |
| Phase | Phase 1 SAD / MAD expansion planned |
| Drug | IBI3011 (anti‑IL‑1RAP mAb) |
| Mechanism | Blocks IL‑1 signaling via IL‑1RAP accessory protein |
| Enrollment | 64 total: 40 healthy volunteers; 24 gout flare patients |
| Primary Endpoints | Safety, tolerability, PK profile, PD biomarkers (IL‑6, CRP) |
| First Dose Date | 09 Dec 2025 |
| Expected Completion | Q4 2026 (SAD); Q2 2027 (MAD) |
| Next Milestone | IND filing for rheumatoid arthritis / CAPS indications H2 2026 |
Drug Profile & Preclinical Evidence
- Novel Target: IL‑1RAP (Interleukin‑1 receptor accessory protein) – a key co‑receptor for IL‑1α/β signaling, implicated in gout, rheumatoid arthritis, CAPS, and other autoinflammatory diseases.
- First‑in‑China: IBI3011 is the first anti‑IL‑1RAP mAb developed in China for inflammatory disease; global competitors include SOBI’s LUTIKIZUMAB (Phase 2) and Novartis’ early‑stage candidates.
- Preclinical Data: In acute gouty arthritis models, IBI3011 demonstrated dose‑dependent suppression of joint swelling and reduced neutrophil infiltration, with ED50 of 0.3 mg/kg – comparable to IL‑1β blockers but with extended PK half‑life (projected 18‑22 days in humans).
- Broader Pipeline Potential: IL‑1RAP inhibition may address gout flare prevention and tumor‑associated inflammation, offering a dual immunology‑oncology platform opportunity.
Market Opportunity & Competitive Landscape
| Metric | Value | Source |
|---|---|---|
| China Gout Prevalence | 15.9 million patients (2024) | China CDC |
| China Gout Treatment Market | ¥3.2 billion (≈ US$450 M) | IQVIA |
| Unmet Need | ~40% of patients experience ≥2 flares/year despite urate‑lowering therapy | |
| Competing Agents | Canakinumab (Ilaris®) – IL‑1β mAb; Anakinra – IL‑1R antagonist; both require frequent dosing and are not IL‑1RAP‑targeted | |
| IBI3011 Value Proposition | Monthly dosing, potentially superior flare prevention, first local biosimilar‑like cost structure |
Innovent projects ¥800 million (≈ US$110 M) peak China sales by 2033 if approved, assuming 12% market share of the refractory gout segment. Global rights could add US$250‑300 M opportunity in EU/U.S. markets currently lacking an IL‑1RAP‑specific therapy.
Forward‑Looking Statements
This brief includes forward‑looking statements regarding IBI3011 clinical progression, market size estimates, and competitive positioning. Actual results may differ due to regulatory delays, unforeseen safety signals, or slower market adoption.-Fineline Info & Tech