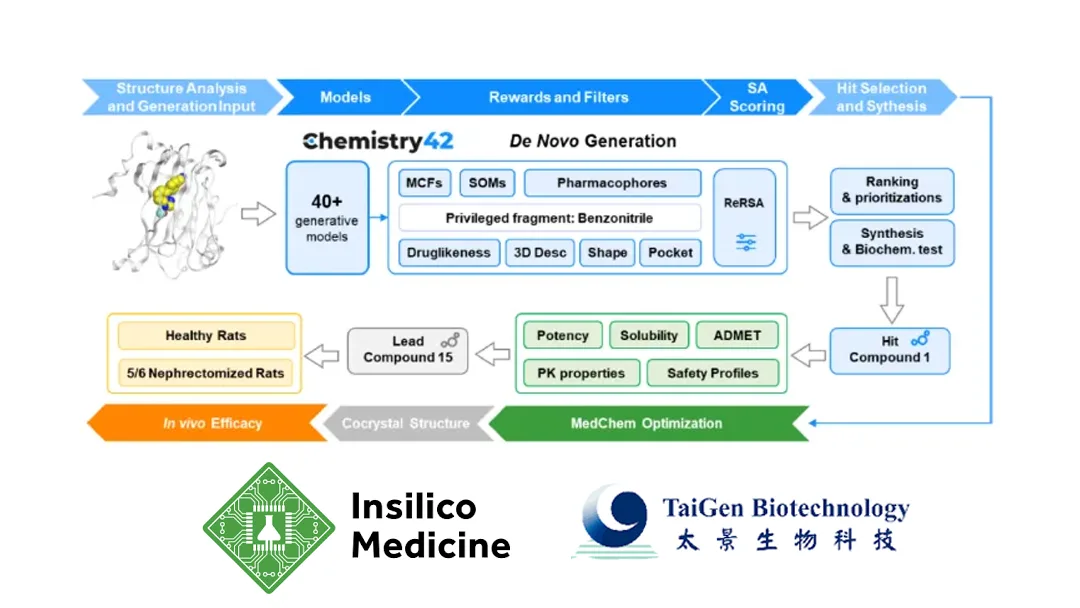
Insilico Medicine and TaiGen Biotechnology (including its Beijing subsidiary TaiGen Biopharmaceuticals) announced an exclusive pipeline out‑licensing collaboration for ISM4808, a potential best‑in‑class oral PHD inhibitor developed via Insilico’s Chemistry42 AI engine. TaiGen gains exclusive rights for development, commercialization, and sub‑licensing in Greater China (Mainland China, Hong Kong, Macau, Taiwan). Insilico is eligible for upfront, development and sales‑based milestone payments, plus tiered royalties, with total deal value reaching the two‑digit million dollar range.
Transaction Structure
| Component | Details |
|---|---|
| Asset Licensed | ISM4808 (oral PHD inhibitor) + related forms/structures |
| Territory | Greater China (Mainland, Hong Kong, Macau, Taiwan) |
| Rights | Exclusive development, commercialization, and sub‑licensing |
| Upfront Payment | One‑time upfront (undisclosed) |
| Milestone Payments | Development and sales‑based milestones |
| Total Deal Value | Two‑digit million USD (estimated $30‑99 M) |
| Royalties | Tiered royalties on net sales |
| IND Status | IND clearance granted in 2023 |
Drug Profile & Differentiation
| Attribute | ISM4808 | Competitive Context |
|---|---|---|
| Mechanism | Oral, small‑molecule PHD inhibitor (prolyl hydroxylase domain) | Modulates HIF pathway for anemia, ischemia, inflammatory diseases |
| Design | AI‑empowered by Insilico’s Chemistry42 engine | Novel scaffold discovered via generative AI vs. traditional HTS |
| Selectivity | Best‑in‑class potential (high PHD selectivity) | Differentiated from earlier pan‑PHD inhibitors (roxadustat, vadadustat) |
| Indications | Undisclosed; likely anemia of chronic disease, ischemic conditions | Expands PHD class beyond CKD anemia |
| Development Stage | IND‑ready (clearance 2023) | Ready for Phase 1 initiation in China H2 2026 |
| Route | Oral (QD dosing) | Patient‑friendly vs. injectable biologics |
Strategic Implications
- For Insilico: Validates AI drug discovery platform with a regional licensing deal; monetizes early‑stage asset while retaining global rights ex‑China; generates non‑dilutive capital to fund broader pipeline.
- For TaiGen: Secures first‑in‑class oral PHD inhibitor for Greater China; leverages established nephrology/immunology sales force; positions for NRDL inclusion in anemia/ischemic disease markets.
- For PHD Market: Global PHD inhibitors generated $3.5 billion in 2024 (CKD anemia); ISM4808’s AI‑designed selectivity could enable broader indications (stroke, peripheral artery disease) representing $5‑7 billion opportunity.
Forward‑Looking Statements
This brief contains forward‑looking statements regarding ISM4808’s development timeline, milestone achievements, and market potential. Actual results may differ due to regulatory delays, competitive responses, or unforeseen safety signals.-Fineline Info & Tech