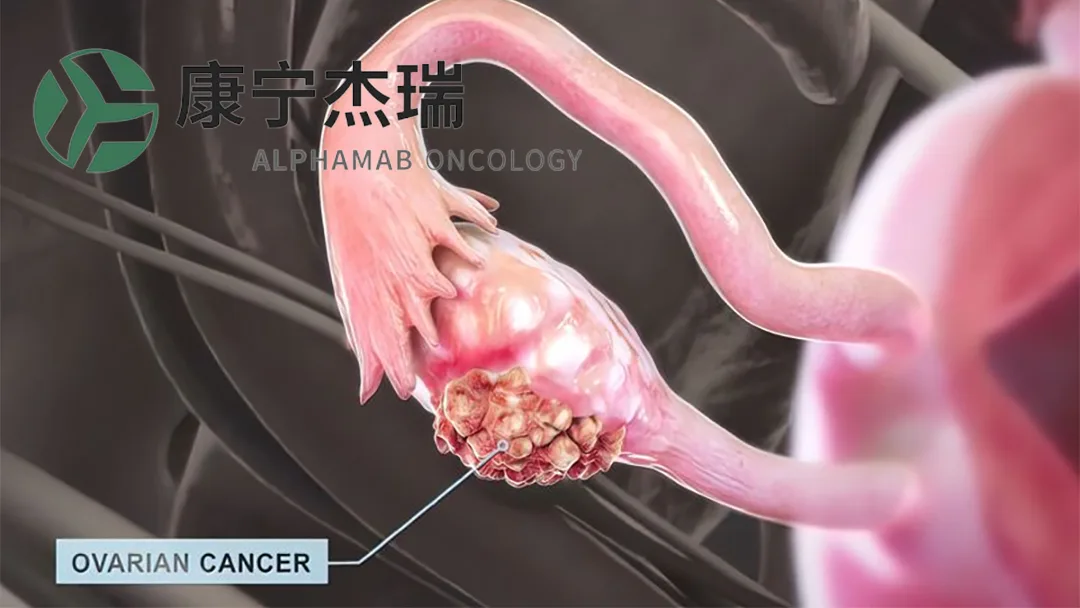
Alphamab Oncology (HKG: 9966) announced that its independently developed HER2‑targeting biparatopic antibody‑drug conjugate (ADC), JSKN003, has been granted Breakthrough Therapy Designation (BTD) by the U.S. Food and Drug Administration (FDA) for the treatment of platinum‑resistant recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer (PROC) with HER2 expression.
Regulatory Milestone & Product Profile
| Attribute | Details |
|---|---|
| Company | Alphamab Oncology (HKG: 9966) |
| Product | JSKN003 |
| Designation | FDA Breakthrough Therapy Designation (BTD) |
| Indication | Advanced/metastatic platinum‑resistant recurrent ovarian/peritoneal/fallopian tube cancer with HER2 expression (IHC 1+, 2+, or 3+) |
| Prior Therapy | Patients must have received prior bevacizumab |
| Drug Class | HER2‑targeting biparatopic ADC |
| Innovation | First biparatopic HER2 ADC with DAR 4; binds two distinct HER2 epitopes |
Mechanism of Action & Technical Differentiation
- Biparatopic Design: Binds to two distinct epitopes of HER2 on tumor cells, enhancing internalization
- ADC Payload: Releases topoisomerase I inhibitor upon internalization
- DAR Optimization: Drug-to-antibody ratio (DAR) of 4 achieved through site‑specific conjugation to Fc glycans of anbenitamab
- Manufacturing: Stable, homogeneous ADC profile for consistent therapeutic effect
Licensing Partnership & Regional Rights
| Agreement Element | Details |
|---|---|
| Counterparty | JMT‑Bio Technology (wholly‑owned subsidiary of CSPC Pharmaceutical Group, 1093.HK) |
| Deal Date | September 2024 |
| Territory | Mainland China (excludes Hong Kong, Macau, Taiwan) |
| Rights | Exclusive license for oncology indications |
| Marketing Authorization Holder | JMT‑Bio serves as sole MAH for oncology indications in Mainland China |
| Manufacturing Rights | Alphamab Oncology retains exclusive manufacturing rights |
Market Context & Strategic Outlook
- PROC Market: 50,000‑70,000 patients in US annually; HER2‑expressing subset represents significant unmet need
- ADC Landscape: HER2 ADCs dominate breast cancer; expansion into ovarian cancer offers $1‑2 billion market opportunity
- Breakthrough Advantage: BTD accelerates FDA review timeline from 10‑12 months to 6‑8 months
- Global Development: US BTD strengthens China regulatory position; pivotal trial initiation planned for Q2 2026
- Revenue Potential: Analysts project $500‑800 million peak global sales if approved across HER2‑expressing solid tumors
Forward‑Looking Statements
This brief contains forward‑looking statements regarding JSKN003 development timelines, clinical outcomes, and partnership execution. Actual results may differ due to clinical, regulatory, and competitive uncertainties.-Fineline Info & Tech