Huadong Medicine Co., Ltd (SHE: 000963), a Chinese pharmaceutical company, has announced that it has received approval from China’s National Medical Products Administration (NMPA) to conduct a study for its Category 1 chemical drug HDM2006, which targets advanced solid tumors. This development marks a significant step for Huadong as it advances its pipeline in oncology.
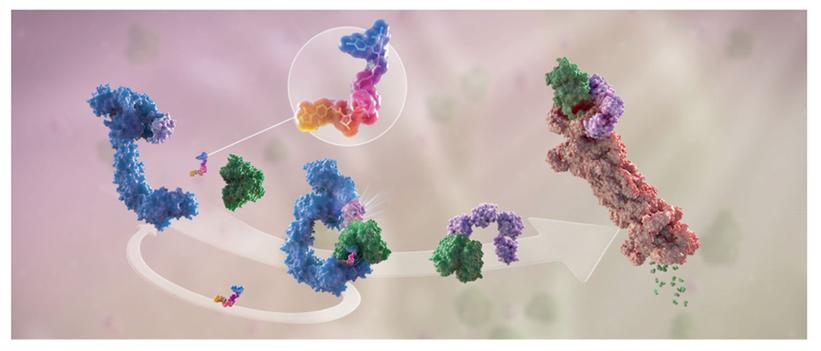
HDM2006 is a novel HPK1 PROteolysis TArgeting Chimera (PROTAC) drug, and Huadong holds global intellectual property rights for this compound. Preclinical study results have demonstrated that HDM2006 possesses favorable drug properties, safety, and efficacy. The drug is designed to specifically bind to human HPK1 and E3 ligases, forming a ternary complex that is subsequently ubiquitinated and targeted for protein degradation. This process alleviates the negative regulation of HPK1 on human T lymphocytes, B lymphocytes, and dendritic cells (DC cells), thereby activating immune cells, reversing immune suppression, and addressing T cell depletion within the tumor microenvironment. These actions contribute to HDM2006’s anti-tumor effects.
The approval to proceed with clinical studies is a validation of Huadong’s commitment to innovation in drug development and could offer new therapeutic options for patients with advanced solid tumors.- Flcube.com