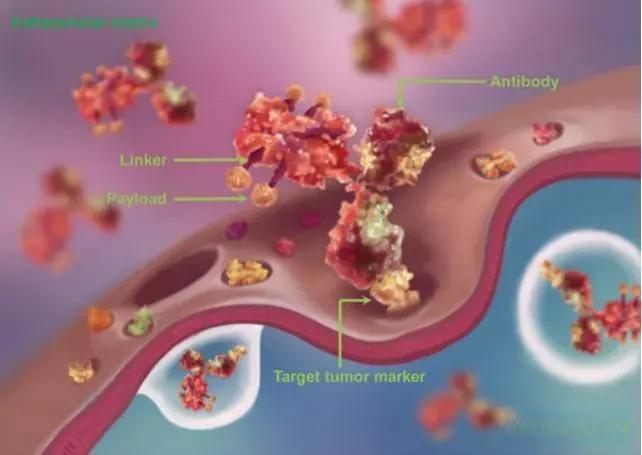
The Center for Drug Evaluation of the National Medical Products Administration (NMPA) has indicated on its website that three antibody drug conjugates (ADCs) from Chinese pharmaceutical companies are on track to receive breakthrough therapy designations (BTDs) in China. These include Shanghai Escugen’s ESG401, Jiangsu Hansoh Pharmaceutical Co., Ltd (HKG: 3692)’s HS-20093 (GSK5764227), and MediLink Therapeutics (Suzhou) Co., Ltd’s YL201.
ESG401, a Trop-2-targeted ADC, has demonstrated significantly higher tolerability than other ADCs in clinical trials, with a low incidence of mild off-target and on-target toxicity, offering significant safety advantages. The BTD status is related to the treatment of irresectable locally advanced, recurrent, or metastatic PD-L1 negative triple negative breast cancer that has not received systematic treatment in the past.
HS-20093 is an innovative B7-H3-targeted ADC consisting of a fully humanized anti-B7-H3 monoclonal antibody linked to a topoisomerase inhibitor (TOPOi) payload. In December 2023, GSK entered into a licensing agreement with Hansoh, securing exclusive global rights to the drug (excluding mainland China, Hong Kong, Macau, and Taiwan) for an upfront payment of USD 185 million and potential future milestone payments of USD 1.525 billion. The BTD award follows a previous BTD status in the US in August this year and is for the treatment of extensive stage small cell lung cancer that has progressed after standard first-line treatment, including platinum dual therapy chemotherapy combined with immunotherapy.
Lastly, YL201, a B7-H3-targeting ADC, has been granted BTD status for the treatment of recurrent small cell lung cancer that has failed initial platinum therapy.- Flcube.com